Structure ( IF 4.4 ) Pub Date : 2021-09-28 , DOI: 10.1016/j.str.2021.09.002 Gabriella Collu 1 , Tobias Bierig 1 , Anna-Sophia Krebs 2 , Sylvain Engilberge 3 , Niveditha Varma 2 , Ramon Guixà-González 4 , Timothy Sharpe 5 , Xavier Deupi 6 , Vincent Olieric 3 , Emiliya Poghosyan 2 , Roger M Benoit 2
|
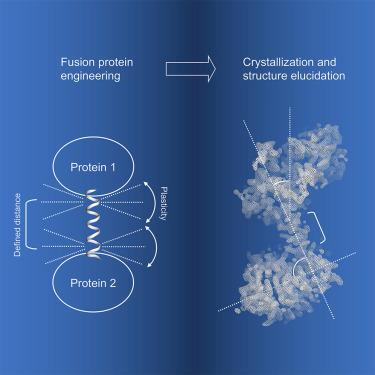
Chimeric fusion proteins are essential tools for protein nanotechnology. Non-optimized protein-protein connections are usually flexible and therefore unsuitable as structural building blocks. Here we show that the ER/K motif, a single α-helical domain (SAH), can be seamlessly fused to terminal helices of proteins, forming an extended, partially free-standing rigid helix. This enables the connection of two domains at a defined distance and orientation. We designed three constructs termed YFPnano, T4Lnano, and MoStoNano. Analysis of experimentally determined structures and molecular dynamics simulations reveals a certain degree of plasticity in the connections that allows the adaptation to crystal contact opportunities. Our data show that SAHs can be stably integrated into designed structural elements, enabling new possibilities for protein nanotechnology, for example, to improve the exposure of epitopes on nanoparticles (structural vaccinology), to engineer crystal contacts with minimal impact on construct flexibility (for the study of protein dynamics), and to design novel biomaterials.
中文翻译:

嵌合单α-螺旋结构域作为蛋白质纳米技术和结构生物学的刚性融合蛋白连接
嵌合融合蛋白是蛋白质纳米技术的重要工具。未优化的蛋白质-蛋白质连接通常是灵活的,因此不适合作为结构构建块。在这里,我们展示了 ER/K 基序,一个单一的 α-螺旋结构域 (SAH),可以无缝融合到蛋白质的末端螺旋上,形成一个延伸的、部分独立的刚性螺旋。这使得两个域能够以定义的距离和方向连接。我们设计了三个结构,称为 YFPnano、T4Lnano 和 MoStoNano。对实验确定的结构和分子动力学模拟的分析揭示了连接中有一定程度的可塑性,可以适应晶体接触机会。我们的数据表明,SAHs 可以稳定地集成到设计的结构元素中,







































 京公网安备 11010802027423号
京公网安备 11010802027423号