当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying Distinct Structural Features of the SARS-CoV-2 Spike Protein Fusion Domain Essential for Membrane Interaction
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00543 Daniel Birtles 1 , Jinwoo Lee 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00543 Daniel Birtles 1 , Jinwoo Lee 1
Affiliation
|
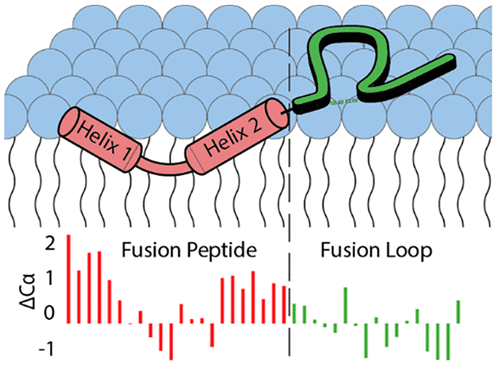
The SARS-CoV-2 spike protein is the primary antigenic determinant of the virus and has been studied extensively, yet the process of membrane fusion remains poorly understood. The fusion domain (FD) of viral glycoproteins is well established as facilitating the initiation of membrane fusion. An improved understanding of the structural plasticity associated with these highly conserved regions aids in our knowledge of the molecular mechanisms that drive viral fusion. Within the spike protein, the FD of SARS-CoV-2 exists immediately following S2′ cleavage at the N-terminus of the S2 domain. Here we have shown that following the introduction of a membrane at pH 7.4, the FD undergoes a transition from a random coil to a more structurally well-defined postfusion state. Furthermore, we have classified the domain into two distinct regions, a fusion peptide (FP, S816–G838) and a fusion loop (FL, D839–F855). The FP forms a helix–turn–helix motif upon association with a membrane, and the favorable entropy gained during this transition from a random coil is likely the driving force behind membrane insertion. Membrane depth experiments then revealed the FP is found inserted within the membrane below the lipid headgroups, while the interaction of the FL with the membrane is shallower in nature. Thus, we propose a structural model relevant to fusion at the plasma membrane in which the FP inserts itself just below the phospholipid headgroups and the FL lays upon the lipid membrane surface.
中文翻译:

识别对膜相互作用至关重要的 SARS-CoV-2 刺突蛋白融合域的独特结构特征
SARS-CoV-2 刺突蛋白是病毒的主要抗原决定簇,已被广泛研究,但膜融合过程仍知之甚少。病毒糖蛋白的融合结构域 (FD) 已被证实可促进膜融合的启动。更好地了解与这些高度保守区域相关的结构可塑性有助于我们了解驱动病毒融合的分子机制。在刺突蛋白中,SARS-CoV-2 的 FD 在 S2 结构域 N 末端的 S2' 切割后立即存在。在这里,我们表明,在引入 pH 7.4 的膜后,FD 经历了从随机卷曲到结构更明确的融合后状态的转变。此外,我们将该结构域分为两个不同的区域:融合肽(FP,S 816 –G 838)和融合环(FL,D 839 –F 855)。FP 在与膜结合时形成螺旋-转角-螺旋基序,并且在从随机卷曲的转变过程中获得的有利熵可能是膜插入背后的驱动力。膜深度实验揭示了 FP 插入到脂质头基下方的膜内,而 FL 与膜的相互作用本质上较浅。因此,我们提出了一个与质膜融合相关的结构模型,其中 FP 将自身插入到磷脂头基下方,而 FL 位于脂质膜表面。
更新日期:2021-10-12
中文翻译:

识别对膜相互作用至关重要的 SARS-CoV-2 刺突蛋白融合域的独特结构特征
SARS-CoV-2 刺突蛋白是病毒的主要抗原决定簇,已被广泛研究,但膜融合过程仍知之甚少。病毒糖蛋白的融合结构域 (FD) 已被证实可促进膜融合的启动。更好地了解与这些高度保守区域相关的结构可塑性有助于我们了解驱动病毒融合的分子机制。在刺突蛋白中,SARS-CoV-2 的 FD 在 S2 结构域 N 末端的 S2' 切割后立即存在。在这里,我们表明,在引入 pH 7.4 的膜后,FD 经历了从随机卷曲到结构更明确的融合后状态的转变。此外,我们将该结构域分为两个不同的区域:融合肽(FP,S 816 –G 838)和融合环(FL,D 839 –F 855)。FP 在与膜结合时形成螺旋-转角-螺旋基序,并且在从随机卷曲的转变过程中获得的有利熵可能是膜插入背后的驱动力。膜深度实验揭示了 FP 插入到脂质头基下方的膜内,而 FL 与膜的相互作用本质上较浅。因此,我们提出了一个与质膜融合相关的结构模型,其中 FP 将自身插入到磷脂头基下方,而 FL 位于脂质膜表面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号