European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-09-28 , DOI: 10.1016/j.ejmech.2021.113871 Xin Xue 1 , Ji-Bo Kang 1 , Xiao Yang 1 , Nan Li 1 , Liang Chang 1 , Juan Ji 2 , Xiang-Kai Meng 1 , Hai-Qing Zhang 1 , Yue Zhong 1 , Shao-Peng Yu 3 , Wen-Yu Wu 1 , Xiao-Long Wang 1 , Nian-Guang Li 1 , Shan-Liang Sun 1
|
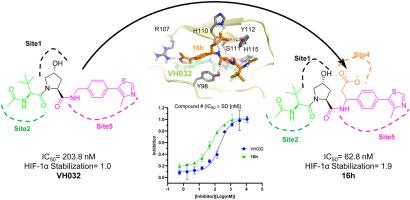
The ubiquitination of the hypoxia-inducible factor-1α (HIF-1α) is mediated by interacting with the von Hippel-Lindau protein (VHL), and is associated with cancer, chronic anemia, and ischemia. VHL, an E3 ligase, has been reported to degrade HIF-1 for decades, however, there are few successful inhibitors currently. Poor understanding of the binding pocket and a lack of in-depth exploration of the interactions between two proteins are the main reasons. Hence, we developed an effective strategy to identify and design new inhibitors for protein-protein interaction targets. The hydroxyproline (Hyp564) of HIF-1α contributed the key interaction between HIF-1α and VHL. In this study, detailed information of the binding pocket were explored by alanine scanning, site-directed mutagenesis and molecular dynamics simulations. Interestingly, we found the interaction(s) between Y565 and H110 played a key role in the binding of VHL/HIF-1α. Based on the interactions, 8 derivates of VH032, 16a-h, were synthesized by introducing various groups bounded to H110. Further assay on protein and cellular level exhibited that 16a-h accessed higher binding affinity to VHL and markable or modest improvement in stabilization of HIF-1α or HIF-1α-OH in HeLa cells. Our work provides a new orientation for the modification or design of VHL/HIF-1α protein-protein interaction inhibitors.
中文翻译:

挖掘蛋白质-蛋白质相互作用以进行合理药物设计的有效策略——以 HIF-1α/VHL 为例
缺氧诱导因子-1α (HIF-1α) 的泛素化是通过与 von Hippel-Lindau 蛋白 (VHL) 相互作用介导的,并且与癌症、慢性贫血和缺血有关。据报道,VHL 是一种 E3 连接酶,几十年来可以降解 HIF-1,然而,目前几乎没有成功的抑制剂。对结合口袋的了解不足以及对两种蛋白质之间的相互作用缺乏深入探索是主要原因。因此,我们开发了一种有效的策略来识别和设计蛋白质-蛋白质相互作用靶点的新抑制剂。HIF-1α 的羟脯氨酸 (Hyp564) 促成了 HIF-1α 和 VHL 之间的关键相互作用。在这项研究中,通过丙氨酸扫描、定点诱变和分子动力学模拟探索了结合口袋的详细信息。有趣的是,我们发现 Y565 和 H110 之间的相互作用在 VHL/HIF-1α 的结合中起关键作用。基于相互作用,VH032 的 8 个衍生物,图 16a-h是通过引入与 H110 绑定的各种基团来合成的。对蛋白质和细胞水平的进一步分析表明,16a-h对 VHL 具有更高的结合亲和力,并且在 HeLa 细胞中 HIF-1α 或 HIF-1α-OH 的稳定性有显着或适度的改善。我们的工作为VHL/HIF-1α蛋白-蛋白相互作用抑制剂的修饰或设计提供了新的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号