当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The characterization of protein interactions – what, how and how much?
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2021-09-28 , DOI: 10.1039/d1cs00548k Louise J Walport 1, 2 , Jason K K Low 3 , Jacqueline M Matthews 3 , Joel P Mackay 3
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2021-09-28 , DOI: 10.1039/d1cs00548k Louise J Walport 1, 2 , Jason K K Low 3 , Jacqueline M Matthews 3 , Joel P Mackay 3
Affiliation
|
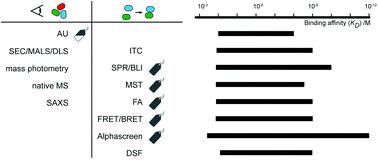
Protein interactions underlie most molecular events in biology. Many methods have been developed to identify protein partners, to measure the affinity with which these biomolecules interact and to characterise the structures of the complexes. Each approach has its own advantages and limitations, and it can be difficult for the newcomer to determine which methodology would best suit their system. This review provides an overview of many of the techniques most widely used to identify protein partners, assess stoichiometry and binding affinity, and determine low-resolution models for complexes. Key methods covered include: yeast two-hybrid analysis, affinity purification mass spectrometry and proximity labelling to identify partners; size-exclusion chromatography, scattering methods, native mass spectrometry and analytical ultracentrifugation to estimate stoichiometry; isothermal titration calorimetry, biosensors and fluorometric methods (including microscale thermophoresis, anisotropy/polarisation, resonance energy transfer, AlphaScreen, and differential scanning fluorimetry) to measure binding affinity; and crosslinking and hydrogen-deuterium exchange mass spectrometry to probe the structure of complexes.
中文翻译:

蛋白质相互作用的表征——什么、如何和多少?
蛋白质相互作用是生物学中大多数分子事件的基础。已经开发了许多方法来识别蛋白质伙伴、测量这些生物分子相互作用的亲和力以及表征复合物的结构。每种方法都有自己的优点和局限性,新手很难确定哪种方法最适合他们的系统。本综述概述了许多最广泛用于识别蛋白质伙伴、评估化学计量和结合亲和力以及确定复合物的低分辨率模型的技术。涵盖的关键方法包括:酵母双杂交分析、亲和纯化质谱和邻近标记以识别合作伙伴;尺寸排阻色谱法,散射方法,天然质谱法和分析超速离心法来估计化学计量;等温滴定量热法、生物传感器和荧光法(包括微型热泳、各向异性/极化、共振能量转移、AlphaScreen 和差示扫描荧光法)来测量结合亲和力;以及交联和氢-氘交换质谱来探测配合物的结构。
更新日期:2021-09-28
中文翻译:

蛋白质相互作用的表征——什么、如何和多少?
蛋白质相互作用是生物学中大多数分子事件的基础。已经开发了许多方法来识别蛋白质伙伴、测量这些生物分子相互作用的亲和力以及表征复合物的结构。每种方法都有自己的优点和局限性,新手很难确定哪种方法最适合他们的系统。本综述概述了许多最广泛用于识别蛋白质伙伴、评估化学计量和结合亲和力以及确定复合物的低分辨率模型的技术。涵盖的关键方法包括:酵母双杂交分析、亲和纯化质谱和邻近标记以识别合作伙伴;尺寸排阻色谱法,散射方法,天然质谱法和分析超速离心法来估计化学计量;等温滴定量热法、生物传感器和荧光法(包括微型热泳、各向异性/极化、共振能量转移、AlphaScreen 和差示扫描荧光法)来测量结合亲和力;以及交联和氢-氘交换质谱来探测配合物的结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号