当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional and protective hole hopping in metalloenzymes
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-27 , DOI: 10.1039/d1sc04286f Harry B Gray 1 , Jay R Winkler 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-27 , DOI: 10.1039/d1sc04286f Harry B Gray 1 , Jay R Winkler 1
Affiliation
|
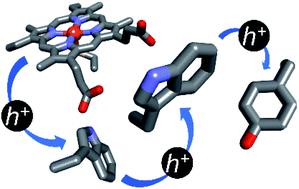
Electrons can tunnel through proteins in microseconds with a modest release of free energy over distances in the 15 to 20 Å range. To span greater distances, or to move faster, multiple charge transfers (hops) are required. When one of the reactants is a strong oxidant, it is convenient to consider the movement of a positively charged “hole” in a direction opposite to that of the electron. Hole hopping along chains of tryptophan (Trp) and tyrosine (Tyr) residues is a critical function in several metalloenzymes that generate high-potential intermediates by reactions with O2 or H2O2, or by activation with visible light. Examination of the protein structural database revealed that Tyr/Trp chains are common protein structural elements, particularly among enzymes that react with O2 and H2O2. In many cases these chains may serve a protective role in metalloenzymes by deactivating high-potential reactive intermediates formed in uncoupled catalytic turnover.
中文翻译:

金属酶中的功能性和保护性空穴跳跃
电子可以在微秒内穿过蛋白质,并在 15 至 20 Å 范围内的距离内适度释放自由能。为了跨越更远的距离,或者移动得更快,需要多次电荷转移(跳跃)。当其中一种反应物是强氧化剂时,可以方便地考虑带正电的“空穴”沿与电子相反的方向移动。沿着色氨酸 (Trp) 和酪氨酸 (Tyr) 残基链的空穴跳跃是几种金属酶的关键功能,这些金属酶通过与 O 2或 H 2 O 2反应或通过可见光激活产生高电位中间体。对蛋白质结构数据库的检查表明,Tyr/Trp 链是常见的蛋白质结构元件,特别是在与 O 2和 H 2 O 2反应的酶中。在许多情况下,这些链可以通过使非偶联催化转换中形成的高电位反应中间体失活,在金属酶中发挥保护作用。
更新日期:2021-09-28
中文翻译:

金属酶中的功能性和保护性空穴跳跃
电子可以在微秒内穿过蛋白质,并在 15 至 20 Å 范围内的距离内适度释放自由能。为了跨越更远的距离,或者移动得更快,需要多次电荷转移(跳跃)。当其中一种反应物是强氧化剂时,可以方便地考虑带正电的“空穴”沿与电子相反的方向移动。沿着色氨酸 (Trp) 和酪氨酸 (Tyr) 残基链的空穴跳跃是几种金属酶的关键功能,这些金属酶通过与 O 2或 H 2 O 2反应或通过可见光激活产生高电位中间体。对蛋白质结构数据库的检查表明,Tyr/Trp 链是常见的蛋白质结构元件,特别是在与 O 2和 H 2 O 2反应的酶中。在许多情况下,这些链可以通过使非偶联催化转换中形成的高电位反应中间体失活,在金属酶中发挥保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号