当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimum in ligand density for conductivity in polymer electrolytes
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2021-09-28 , DOI: 10.1039/d1me00089f Nicole S. Schauser 1, 2, 3 , Peter M. Richardson 2, 3 , Andrei Nikolaev 3, 4 , Piper Cooke 2, 4 , Gabrielle A. Kliegle 2, 4 , Ethan M. Susca 2 , Keith Johnson 1, 2 , Hengbin Wang 3 , Javier Read de Alaniz 4 , Raphaële Clément 1, 2, 3 , Rachel A. Segalman 1, 2, 3, 5
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2021-09-28 , DOI: 10.1039/d1me00089f Nicole S. Schauser 1, 2, 3 , Peter M. Richardson 2, 3 , Andrei Nikolaev 3, 4 , Piper Cooke 2, 4 , Gabrielle A. Kliegle 2, 4 , Ethan M. Susca 2 , Keith Johnson 1, 2 , Hengbin Wang 3 , Javier Read de Alaniz 4 , Raphaële Clément 1, 2, 3 , Rachel A. Segalman 1, 2, 3, 5
Affiliation
|
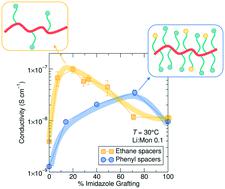
Current design rules for ion conducting polymers suggest that fast segmental dynamics and high solvation site density are important for high performance. In a family of imidazole side chain grafted siloxane polymer electrolytes containing LiTFSI, we conclude that while the presence of imidazole solvation sites promotes solubilization of Li+ containing salts, it is not necessary to substitute every monomer in the polymer design. Rather, optimization of Li+ conductivity relies on a balance between imidazole presence and the ability of the chains to rearrange locally to facilitate transport. Lowering the imidazole content in the ethane-imidazole series leads to a 10-fold increase in conductivity, while conductivity decreases for the phenyl-imidazole series due to differences in steric bulk. Normalizing conductivity by Tg reveals a threshold ligand density above which increased solvation sites do not improve conductivity, but below which the conduction gradually decreases. NMR spectroscopy shows the high temperature Li+ transport number increases slightly with increasing grafting density, from around 0.17 to 0.24. NMR T1ρ relaxation reveals that the Li+ ions are present in two environments with distinct dynamics within the polymer, matching X-ray scattering and PFG results which suggest ion aggregation exists in these polymers. These results emphasize the importance of local re-arrangements in facilitating ion transport at low solvation site density, confirming the role of dynamic percolation, and suggest that an optimum ligand density exists for improved charge transport.
中文翻译:

聚合物电解质中电导率的最佳配体密度
当前离子导电聚合物的设计规则表明,快速的链段动力学和高溶剂化位点密度对于高性能很重要。在含有 LiTFSI 的咪唑侧链接枝硅氧烷聚合物电解质家族中,我们得出结论,虽然咪唑溶剂化位点的存在促进了含Li +盐的溶解,但没有必要在聚合物设计中替换每个单体。相反,优化 Li +电导率取决于咪唑的存在与链局部重新排列以促进运输的能力之间的平衡。降低乙烷-咪唑系列中的咪唑含量会导致电导率增加 10 倍,而苯基-咪唑系列的电导率由于空间体积的差异而降低。通过T g归一化电导率揭示了阈值配体密度,高于该阈值增加的溶剂化位点不会提高电导率,但低于该阈值则电导率逐渐降低。NMR 光谱显示高温 Li +传输数随着接枝密度的增加而略有增加,从大约 0.17 到 0.24。NMR T 1 ρ弛豫表明Li +离子存在于聚合物内具有不同动力学的两种环境中,匹配 X 射线散射和 PFG 结果,表明这些聚合物中存在离子聚集。这些结果强调了局部重排在低溶剂化位点密度下促进离子传输的重要性,证实了动态渗透的作用,并表明存在最佳配体密度以改善电荷传输。
更新日期:2021-09-28
中文翻译:

聚合物电解质中电导率的最佳配体密度
当前离子导电聚合物的设计规则表明,快速的链段动力学和高溶剂化位点密度对于高性能很重要。在含有 LiTFSI 的咪唑侧链接枝硅氧烷聚合物电解质家族中,我们得出结论,虽然咪唑溶剂化位点的存在促进了含Li +盐的溶解,但没有必要在聚合物设计中替换每个单体。相反,优化 Li +电导率取决于咪唑的存在与链局部重新排列以促进运输的能力之间的平衡。降低乙烷-咪唑系列中的咪唑含量会导致电导率增加 10 倍,而苯基-咪唑系列的电导率由于空间体积的差异而降低。通过T g归一化电导率揭示了阈值配体密度,高于该阈值增加的溶剂化位点不会提高电导率,但低于该阈值则电导率逐渐降低。NMR 光谱显示高温 Li +传输数随着接枝密度的增加而略有增加,从大约 0.17 到 0.24。NMR T 1 ρ弛豫表明Li +离子存在于聚合物内具有不同动力学的两种环境中,匹配 X 射线散射和 PFG 结果,表明这些聚合物中存在离子聚集。这些结果强调了局部重排在低溶剂化位点密度下促进离子传输的重要性,证实了动态渗透的作用,并表明存在最佳配体密度以改善电荷传输。









































 京公网安备 11010802027423号
京公网安备 11010802027423号