Chem Catalysis ( IF 11.5 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.checat.2021.08.006 Yeqin Guan 1, 2 , Weijin Zhang 1, 2 , Qianru Wang 1, 2 , Claudia Weidenthaler 3 , Anan Wu 4 , Wenbo Gao 1, 2 , Qijun Pei 1 , Hanxue Yan 1, 2 , Jirong Cui 1, 2 , Han Wu 1, 2 , Sheng Feng 1, 2 , Runze Wang 1, 2 , Hujun Cao 1, 2 , Xiaohua Ju 1 , Lin Liu 1, 2 , Teng He 1, 2 , Jianping Guo 1, 2 , Ping Chen 1, 2
|
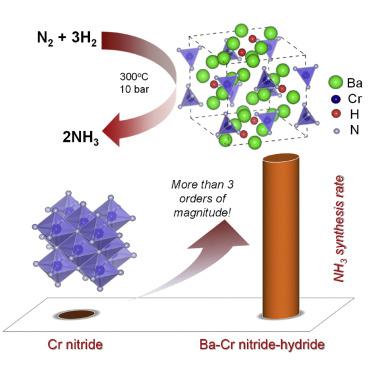
Early 3d metals such as chromium can easily dissociate N2, but the subsequent hydrogenation to ammonia is difficult because they bind nitrogen too strongly. Hence, investigation of Cr-based catalysts for ammonia synthesis is very rare. Here we show that when Cr compounds with Ba, N, and H forming a nitride-hydride, effective ammonia synthesis catalysis can be achieved under mild conditions. Under 573 K and 10 bar, this catalyst has an ammonia synthesis rate (6.8 mmolNH3 gcat−1 h−1) that is about four times that of the Cs-Ru/MgO catalyst. With low apparent activation energy (50.1 kJ mol−1) and positive reaction orders of H2 and N2, it can produce observable ammonia at 373 K and 1 bar. The active phase has a Ba5CrN4H-like structure containing reactive hydrogen () and nitrogen, which are involved in the ammonia formation. This work discloses a strategy to “activate” the inactive early transition metals for effective ammonia catalysis.
中文翻译:

用于氨合成的氮化铬钡
早期的 3d 金属如铬可以很容易地离解 N 2,但随后的氢化成氨很困难,因为它们与氮结合太强。因此,对用于氨合成的 Cr 基催化剂的研究非常罕见。在这里,我们表明当 Cr 与 Ba、N 和 H 化合物形成氮化物氢化物时,可以在温和条件下实现有效的氨合成催化。在 573 K 和 10 bar 下,该催化剂的氨合成速率(6.8 mmol NH3 g cat -1 h -1)约为 Cs-Ru/MgO 催化剂的四倍。具有低表观活化能 (50.1 kJ mol -1 ) 和 H 2和 N 2 的正反应顺序,它可以在 373 K 和 1 bar 下产生可观察到的氨。活性相具有类似 Ba 5 CrN 4 H 的结构,含有活性氢() 和氮,它们参与氨的形成。这项工作揭示了一种“激活”非活性早期过渡金属以实现有效氨催化的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号