当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mono-/Bimetallic Neutral Iridium(III) Complexes Bearing Diketopyrrolopyrrole-Substituted N-Heterocyclic Carbene Ligands: Synthesis and Photophysics
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-28 , DOI: 10.1021/acs.inorgchem.1c01848 Wan Xu 1, 2 , Levi Lystrom 1 , Yanxiong Pan 1 , Xinyang Sun 1 , Salim A Thomas 3 , Svetlana V Kilina 1 , Zhongyu Yang 1 , Hua Wang 2 , Erik K Hobbie 3, 4, 5 , Wenfang Sun 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-28 , DOI: 10.1021/acs.inorgchem.1c01848 Wan Xu 1, 2 , Levi Lystrom 1 , Yanxiong Pan 1 , Xinyang Sun 1 , Salim A Thomas 3 , Svetlana V Kilina 1 , Zhongyu Yang 1 , Hua Wang 2 , Erik K Hobbie 3, 4, 5 , Wenfang Sun 1
Affiliation
|
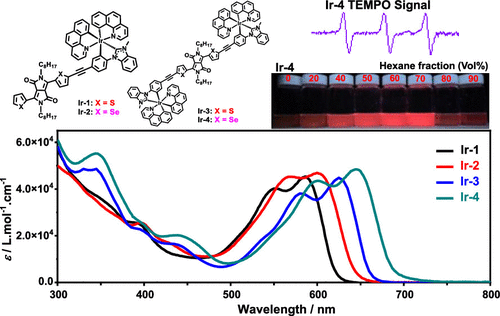
The synthesis and photophysics (UV–vis absorption, emission, and transient absorption) of four neutral heteroleptic cyclometalated iridium(III) complexes (Ir-1–Ir-4) incorporating thiophene/selenophene-diketopyrrolopyrrole (DPP)-substituted N-heterocyclic carbene (NHC) ancillary ligands are reported. The effects of thiophene versus selenophene substitution on DPP and bis- versus monoiridium(III) complexation on the photophysics of these complexes were systematically investigated via spectroscopic techniques and density functional theory calculations. All complexes exhibited strong vibronically resolved absorption in the regions of 500–700 nm and fluorescence at 600–770 nm, and both are predominantly originated from the DPP-NHC ligand. Complexation induced a pronounced red shift of this low-energy absorption band and the fluorescence band with respect to their corresponding ligands due to the improved planarity and extended π-conjugation in the DPP-NHC ligand. Replacing the thiophene units by selenophenes and/or biscomplexation led to the red-shifted absorption and fluorescence spectra, accompanied by the reduced fluorescence lifetime and quantum yield and enhanced population of the triplet excited states, as reflected by the stronger triplet excited-state absorption and singlet oxygen generation.
中文翻译:

带有二酮吡咯并吡咯取代的 N-杂环卡宾配体的单/双金属中性铱 (III) 配合物:合成和光物理学
四种中性杂配环金属化铱(III)配合物(Ir - 1-Ir-4)的合成和光物理(UV-vis吸收、发射和瞬态吸收)) 结合了噻吩/硒吩-二酮吡咯并吡咯 (DPP)-取代的 N-杂环卡宾 (NHC) 辅助配体。通过光谱技术和密度泛函理论计算系统地研究了噻吩与硒吩取代对 DPP 和双-与单铱 (III) 络合对这些配合物光物理的影响。所有配合物在 500-700 nm 区域都表现出强烈的振动分辨吸收,在 600-770 nm 区域显示出荧光,并且两者都主要来自 DPP-NHC 配体。由于 DPP-NHC 配体中平面性的改善和 π 共轭的扩展,络合会导致该低能量吸收带和荧光带相对于其相应配体发生明显的红移。
更新日期:2021-10-18
中文翻译:

带有二酮吡咯并吡咯取代的 N-杂环卡宾配体的单/双金属中性铱 (III) 配合物:合成和光物理学
四种中性杂配环金属化铱(III)配合物(Ir - 1-Ir-4)的合成和光物理(UV-vis吸收、发射和瞬态吸收)) 结合了噻吩/硒吩-二酮吡咯并吡咯 (DPP)-取代的 N-杂环卡宾 (NHC) 辅助配体。通过光谱技术和密度泛函理论计算系统地研究了噻吩与硒吩取代对 DPP 和双-与单铱 (III) 络合对这些配合物光物理的影响。所有配合物在 500-700 nm 区域都表现出强烈的振动分辨吸收,在 600-770 nm 区域显示出荧光,并且两者都主要来自 DPP-NHC 配体。由于 DPP-NHC 配体中平面性的改善和 π 共轭的扩展,络合会导致该低能量吸收带和荧光带相对于其相应配体发生明显的红移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号