当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Significant Insight into the Origin of Reaction Barriers Determining Dihydrogen Activation by G13-P-P (G13 = Group 13 Element) and G15-P-Ga (G15 = Group 15 Element) Frustrated Lewis Pairs
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.inorgchem.1c01809 Zheng-Feng Zhang, Ming-Chung Yang, Ming-Der Su
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.inorgchem.1c01809 Zheng-Feng Zhang, Ming-Chung Yang, Ming-Der Su
|
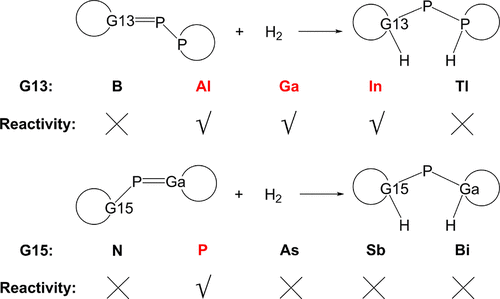
The heterolytic cleavage of H2 by multiply bonded phosphorus-bridged G13-P-P-Rea (G13 = B, Al, Ga, In, and Tl) and G15-P-Ga-Rea (G15 = N, P, As, Sb, and Bi) frustrated Lewis pairs (FLPs) has been theoretically investigated using density functional theory calculations. For the above nine FLP-type molecules, our theoretical findings suggest that only Al-P-P-Rea, Ga-P-P-Rea, and In-P-P-Rea can undergo the energetically feasible H2 activation reaction from kinetic and thermodynamic viewpoints. Our study based on the activation strain model (ASM) reveals that gaining a better orbital overlap between G13-P-P-Rea and G15-P-Ga-Rea molecules and H2 affected the reaction barriers through the atomic radius of G13 and G15. According to our energy decomposition analysis-natural orbitals for chemical valence (EDA-NOCV) results, the bonding of these H2 activation reactions involving G13-P-P-Rea and G15-P-Ga-Rea is dominated by the donor–acceptor interaction (singlet–singlet interaction) rather than the electron-sharing interaction (triplet–triplet interaction). Moreover, our EDA-NOCV evidence reveals that the best description for the above bonding situations is the lone pair(G15) → σ*(H2) interaction rather than the empty p−π-orbital(G13) ← σ(H2) interaction. In particular, the findings in this work based on theoretically calculated geometries and the corresponding relative free energies of the stationary points combined with the results from the above sophisticated methods nicely agree with the famous Hammond postulate.
中文翻译:

对 G13-PP(G13 = 第 13 族元素)和 G15-P-Ga(G15 = 第 15 族元素)受阻路易斯对确定双氢活化的反应障碍起源的重要见解
H 2通过多重键合的磷桥连G13-PP-Rea(G13 = B、Al、Ga、In 和 Tl)和G15-P-Ga-Rea(G15 = N、P、As、Sb,和 Bi) 受挫刘易斯对 (FLP) 已使用密度泛函理论计算进行了理论上的研究。对于上述九种 FLP 型分子,我们的理论发现表明,从动力学和热力学的角度来看,只有Al-PP-Rea、Ga-PP-Rea和In-PP-Rea可以进行能量上可行的 H 2活化反应。我们基于激活应变模型 (ASM) 的研究表明,在G13-PP-Rea和G15-P-Ga-Rea分子和H 2通过G13 和G15 的原子半径影响反应势垒。根据我们的能量分解分析-化学价的自然轨道 (EDA-NOCV) 结果,这些涉及G13-PP-Rea和G15-P-Ga-Rea 的H 2活化反应的键合主要受供体-受体相互作用(单线态-单线态相互作用)而不是电子共享相互作用(三线态-三线态相互作用)。此外,我们的 EDA-NOCV 证据表明,上述键合情况的最佳描述是孤对 (G15) → σ*(H 2 ) 相互作用,而不是空的 p−π-轨道 (G13) ← σ(H 2) 相互作用。特别是,这项工作基于理论计算的几何形状和相应的静止点相对自由能,结合上述复杂方法的结果,与著名的哈蒙德假设非常吻合。
更新日期:2021-10-18
中文翻译:

对 G13-PP(G13 = 第 13 族元素)和 G15-P-Ga(G15 = 第 15 族元素)受阻路易斯对确定双氢活化的反应障碍起源的重要见解
H 2通过多重键合的磷桥连G13-PP-Rea(G13 = B、Al、Ga、In 和 Tl)和G15-P-Ga-Rea(G15 = N、P、As、Sb,和 Bi) 受挫刘易斯对 (FLP) 已使用密度泛函理论计算进行了理论上的研究。对于上述九种 FLP 型分子,我们的理论发现表明,从动力学和热力学的角度来看,只有Al-PP-Rea、Ga-PP-Rea和In-PP-Rea可以进行能量上可行的 H 2活化反应。我们基于激活应变模型 (ASM) 的研究表明,在G13-PP-Rea和G15-P-Ga-Rea分子和H 2通过G13 和G15 的原子半径影响反应势垒。根据我们的能量分解分析-化学价的自然轨道 (EDA-NOCV) 结果,这些涉及G13-PP-Rea和G15-P-Ga-Rea 的H 2活化反应的键合主要受供体-受体相互作用(单线态-单线态相互作用)而不是电子共享相互作用(三线态-三线态相互作用)。此外,我们的 EDA-NOCV 证据表明,上述键合情况的最佳描述是孤对 (G15) → σ*(H 2 ) 相互作用,而不是空的 p−π-轨道 (G13) ← σ(H 2) 相互作用。特别是,这项工作基于理论计算的几何形状和相应的静止点相对自由能,结合上述复杂方法的结果,与著名的哈蒙德假设非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号