当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Finding Ways to Relax: A Revisionistic Analysis of the Chemistry of E. coli GTP Cyclohydrolase II
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00511 Madison M Smith 1 , Brett A Beaupre 1 , Dariush C Fourozesh 1 , Kathleen M Meneely 2 , Audrey L Lamb 2 , Graham R Moran 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00511 Madison M Smith 1 , Brett A Beaupre 1 , Dariush C Fourozesh 1 , Kathleen M Meneely 2 , Audrey L Lamb 2 , Graham R Moran 1
Affiliation
|
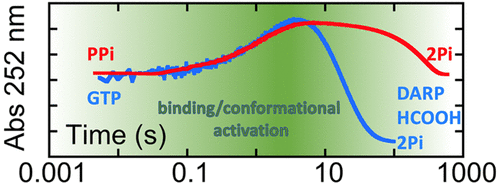
Guanosine triphosphate (GTP) cyclohydrolase II (RibA) is one of three enzymes that hydrolytically cleave the C8–N9 bond of the GTP guanine. RibA also catalyzes a subsequent hydrolytic attack at the base liberating formate and in addition cleaves the α–β phosphodiester bond of the triphosphate to form pyrophosphate (PPi). These hydrolytic reactions are promoted by tandem active-site metal ions, zinc and magnesium, that respectively function at the GTP guanine and triphosphate moieties. The RibA reaction is part of riboflavin biosynthesis and forms 2,5-diamino-6-β-pyrimidinone 5′-phosphate, an exocyclic pyrimidine nucleotide that ultimately forms the pyrimidine ring of the isoalloxazine of riboflavin. The stoichiometry of the RibA reaction was defined in the study that first identified this activity in Escherichia coli (Foor, F., Brown, G. M. J. Biol. Chem., 1975, 250, 9, 3545−3551) and has not been quantitatively evaluated in subsequent works. Using primarily transient state approaches we examined the interaction of RibA from E. coli with the GTP, inosine triphosphate, and PPi. Our data indicate that PPi is a slow substrate for RibA that is cleaved to form two phosphate ions (Pi). A combination of real-time enzymatically coupled Pi reporter assays and end-point 31P NMR revealed that Pi is formed at a catalytically relevant rate in the native reaction of RibA with GTP, redefining the reaction stoichiometry. Furthermore, our data indicate that both PPi and GTP stimulate conformational changes prior to hydrolytic chemistry, and we conclude that the cleavage of PPi bound as a substrate or an intermediate state results in conformational relaxation.
中文翻译:

寻找放松的方法:对大肠杆菌 GTP 环化水解酶 II 化学的修正分析
三磷酸鸟苷 (GTP) 环化水解酶 II (RibA) 是三种酶之一,可水解裂解 GTP 鸟嘌呤的 C8-N9 键。RibA 还催化随后在释放甲酸盐的碱基处发生水解攻击,此外还裂解三磷酸的 α-β 磷酸二酯键以形成焦磷酸盐 (PPi)。这些水解反应由串联活性位点金属离子、锌和镁促进,它们分别在 GTP 鸟嘌呤和三磷酸部分起作用。RibA 反应是核黄素生物合成的一部分,形成 2,5-二氨基-6-β-嘧啶酮 5'-磷酸,这是一种外环嘧啶核苷酸,最终形成核黄素异恶嗪的嘧啶环。RibA 反应的化学计量是在研究中定义的,该研究首先在大肠杆菌中发现了这种活性 (Foor, F. , Brown, GM J.生物。化学 , 1975 , 250 , 9, 3545−3551) 并且没有在后续工作中进行定量评估。我们主要使用瞬态方法检查了来自大肠杆菌的 RibA与 GTP、肌苷三磷酸和 PPi的相互作用。我们的数据表明 PPi 是 RibA 的缓慢底物,它被裂解形成两个磷酸根离子 (Pi)。实时酶联 Pi 报告基因检测和终点31 的组合P NMR 显示,在 RibA 与 GTP 的天然反应中,Pi 以催化相关的速率形成,重新定义了反应的化学计量。此外,我们的数据表明 PPi 和 GTP 在水解化学之前都会刺激构象变化,我们得出结论,作为底物或中间状态结合的 PPi 的裂解导致构象松弛。
更新日期:2021-10-12
中文翻译:

寻找放松的方法:对大肠杆菌 GTP 环化水解酶 II 化学的修正分析
三磷酸鸟苷 (GTP) 环化水解酶 II (RibA) 是三种酶之一,可水解裂解 GTP 鸟嘌呤的 C8-N9 键。RibA 还催化随后在释放甲酸盐的碱基处发生水解攻击,此外还裂解三磷酸的 α-β 磷酸二酯键以形成焦磷酸盐 (PPi)。这些水解反应由串联活性位点金属离子、锌和镁促进,它们分别在 GTP 鸟嘌呤和三磷酸部分起作用。RibA 反应是核黄素生物合成的一部分,形成 2,5-二氨基-6-β-嘧啶酮 5'-磷酸,这是一种外环嘧啶核苷酸,最终形成核黄素异恶嗪的嘧啶环。RibA 反应的化学计量是在研究中定义的,该研究首先在大肠杆菌中发现了这种活性 (











































 京公网安备 11010802027423号
京公网安备 11010802027423号