Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.jclepro.2021.129115 Qi Meng 1 , Xiaohui Yan 1 , Guichun Li 2
|
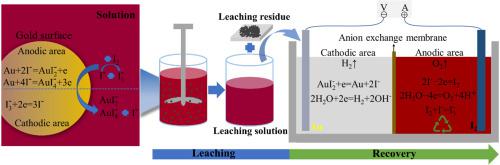
Although iodination gold leaching is a clean, non-toxic and efficient non-cyanide gold leaching process, it still has the problems of high reagents cost and difficult leaching solution treatment. To solve these problems, a process of iodination leaching-electrodeposition recovery was proposed. Thermodynamic analysis confirmed the feasibility of iodination gold leaching, and the influence of different factors on gold leaching was studied. The result shows that roasting could effectively improve the gold leaching effect, the concentration of initial iodine and initial iodide ion had a significant effect on the gold leaching, and the gold leaching could be achieved under normal temperature and neutral conditions. The kinetic analysis shows that the gold leaching process conforms to the shrinkage kinetics model, and the control step was the solid film diffusion control. The apparent activation energy of this reaction was 8.642 kJ/mol, and the reaction order of initial iodine concentration and initial iodide ion concentration were 0.72 and 0.52, respectively. Gold was recovered from the leaching solution by electrodeposition with a recovery percentage of more than 99%. More importantly, the iodine was formed in the anode area, in other words, the reagents used for gold leaching were enriched in the anode area during the gold recovery process. The recovery percentage of iodine in the anode area reached 83%, and the anolyte could be recycled. This process fundamentally solves the problem of high cost of iodination leaching.
中文翻译:

碘化浸出-电沉积回收环保型试剂可回收金提取
碘化浸金虽然是一种清洁、无毒、高效的无氰浸金工艺,但仍存在试剂成本高、浸出液处理困难的问题。针对这些问题,提出了碘化浸出-电沉积回收工艺。热力学分析证实了碘化浸金的可行性,并研究了不同因素对浸金的影响。结果表明,焙烧可有效提高浸金效果,初始碘浓度和初始碘离子浓度对浸金效果显着,可在常温中性条件下实现浸金。动力学分析表明金浸出过程符合收缩动力学模型,控制步骤为固体膜扩散控制。该反应的表观活化能为8.642 kJ/mol,初始碘浓度和初始碘离子浓度的反应阶数分别为0.72和0.52。采用电沉积法从浸出液中回收金,回收率达99%以上。更重要的是,碘是在阳极区形成的,换句话说,在金回收过程中,用于浸金的试剂在阳极区富集。阳极区碘的回收率达到83%,阳极液可回收利用。该工艺从根本上解决了碘化浸出成本高的问题。初始碘浓度和初始碘离子浓度的反应阶数分别为0.72和0.52。采用电沉积法从浸出液中回收金,回收率达99%以上。更重要的是,碘是在阳极区形成的,换句话说,在金回收过程中,用于浸金的试剂在阳极区富集。阳极区碘的回收率达到83%,阳极液可回收利用。该工艺从根本上解决了碘化浸出成本高的问题。初始碘浓度和初始碘离子浓度的反应阶数分别为0.72和0.52。采用电沉积法从浸出液中回收金,回收率达99%以上。更重要的是,碘是在阳极区形成的,换句话说,在金回收过程中,用于浸金的试剂在阳极区富集。阳极区碘的回收率达到83%,阳极液可回收利用。该工艺从根本上解决了碘化浸出成本高的问题。在金回收过程中,用于浸金的试剂在阳极区域富集。阳极区碘的回收率达到83%,阳极液可回收利用。该工艺从根本上解决了碘化浸出成本高的问题。在金回收过程中,用于浸金的试剂在阳极区域富集。阳极区碘的回收率达到83%,阳极液可回收利用。该工艺从根本上解决了碘化浸出成本高的问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号