当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A rotamer relay information system in the epidermal growth factor receptor–drug complexes reveals clues to new paradigm in protein conformational change
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.csbj.2021.09.026 Tareq Hameduh 1 , Michal Mokry 1, 2 , Andrew D Miller 1, 3, 4 , Vojtech Adam 1, 2 , Zbynek Heger 1, 2 , Yazan Haddad 1, 2
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.csbj.2021.09.026 Tareq Hameduh 1 , Michal Mokry 1, 2 , Andrew D Miller 1, 3, 4 , Vojtech Adam 1, 2 , Zbynek Heger 1, 2 , Yazan Haddad 1, 2
Affiliation
|
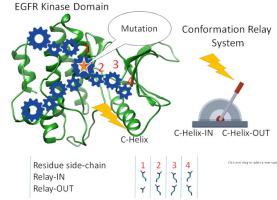
Cancer cells can escape the effects of chemotherapy through mutations and upregulation of a tyrosine kinase protein called the epidermal growth factor receptor (EGFR). In the past two decades, four generations of tyrosine kinase inhibitors targeting EGFR have been developed. Using comparative structure analysis of 116 EGFR-drug complex crystal structures, cluster analysis produces two clans of 73 and 43 structures, respectively. The first clan of 73 structures is larger and is comprised mostly of the C-helix-IN conformation while the second clan of 43 structures correlates with the C-helix-OUT conformation. A deep rotamer analysis identifies 43 residues (18%) of the total of 237 residues spanning the kinase structures under investigation with significant rotamer variations between the C-helix-IN and C-helix-OUT clans. The locations of these rotamer variations take on the appearance of side chain conformational relays extending out from points of EGFR mutation to different regions of the EGFR kinase. Accordingly, we propose that key EGFR mutations act singly or together to induce drug resistant conformational changes in EGFR that are communicated these side chain conformational relays. Accordingly, these side chain conformational relays appear to play a significant role in the development of tumour resistance. This phenomenon also suggests a new paradigm in protein conformational change that is mediated by supportive relays of rotamers on the protein surface, rather than through conventional backbone movements.
中文翻译:

表皮生长因子受体-药物复合物中的旋转异构体中继信息系统揭示了蛋白质构象变化新范式的线索
癌细胞可以通过称为表皮生长因子受体(EGFR)的酪氨酸激酶蛋白的突变和上调来逃避化疗的影响。近二十年来,针对EGFR的酪氨酸激酶抑制剂已开发出四代。通过对 116 种 EGFR-药物复合晶体结构进行比较结构分析,聚类分析分别产生了 73 种和 43 种结构的两个家族。第一个族的 73 个结构较大,主要由 C-helix-IN 构象组成,而第二个族的 43 个结构与 C-helix-OUT 构象相关。深度旋转异构体分析鉴定了所研究的激酶结构中总共 237 个残基中的 43 个残基 (18%),其中 C-helix-IN 和 C-helix-OUT 家族之间存在显着的旋转异构体变异。这些旋转异构体变异的位置呈现侧链构象中继的外观,从 EGFR 突变点延伸到 EGFR 激酶的不同区域。因此,我们提出关键的 EGFR 突变单独或共同作用,诱导 EGFR 的耐药构象变化,这些变化通过这些侧链构象中继传递。因此,这些侧链构象中继似乎在肿瘤抗性的发展中发挥着重要作用。这种现象还表明蛋白质构象变化的新范式是由蛋白质表面旋转异构体的支持性中继介导的,而不是通过传统的主干运动介导。
更新日期:2021-09-27
中文翻译:

表皮生长因子受体-药物复合物中的旋转异构体中继信息系统揭示了蛋白质构象变化新范式的线索
癌细胞可以通过称为表皮生长因子受体(EGFR)的酪氨酸激酶蛋白的突变和上调来逃避化疗的影响。近二十年来,针对EGFR的酪氨酸激酶抑制剂已开发出四代。通过对 116 种 EGFR-药物复合晶体结构进行比较结构分析,聚类分析分别产生了 73 种和 43 种结构的两个家族。第一个族的 73 个结构较大,主要由 C-helix-IN 构象组成,而第二个族的 43 个结构与 C-helix-OUT 构象相关。深度旋转异构体分析鉴定了所研究的激酶结构中总共 237 个残基中的 43 个残基 (18%),其中 C-helix-IN 和 C-helix-OUT 家族之间存在显着的旋转异构体变异。这些旋转异构体变异的位置呈现侧链构象中继的外观,从 EGFR 突变点延伸到 EGFR 激酶的不同区域。因此,我们提出关键的 EGFR 突变单独或共同作用,诱导 EGFR 的耐药构象变化,这些变化通过这些侧链构象中继传递。因此,这些侧链构象中继似乎在肿瘤抗性的发展中发挥着重要作用。这种现象还表明蛋白质构象变化的新范式是由蛋白质表面旋转异构体的支持性中继介导的,而不是通过传统的主干运动介导。










































 京公网安备 11010802027423号
京公网安备 11010802027423号