当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Chemical Study of a Radical Relay Mechanism for the HydG-Catalyzed Synthesis of a Fe(II)(CO)2(CN)cysteine Precursor to the H-Cluster of [FeFe] Hydrogenase
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00379 Nanhao Chen 1 , Guodong Rao 1 , R David Britt 1 , Lee-Ping Wang 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.biochem.1c00379 Nanhao Chen 1 , Guodong Rao 1 , R David Britt 1 , Lee-Ping Wang 1
Affiliation
|
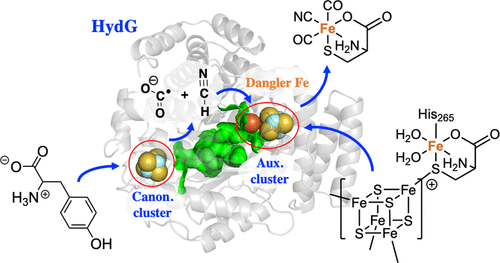
The [FeFe] hydrogenase catalyzes the redox interconversion of protons and H2 with a Fe–S “H-cluster” employing CO, CN, and azadithiolate ligands to two Fe centers. The biosynthesis of the H-cluster is a highly interesting reaction carried out by a set of Fe–S maturase enzymes called HydE, HydF, and HydG. HydG, a member of the radical S-adenosylmethionine (rSAM) family, converts tyrosine, cysteine, and Fe(II) into an organometallic Fe(II)(CO)2(CN)cysteine “synthon”, which serves as the substrate for HydE. Although key aspects of the HydG mechanism have been experimentally determined via isotope-sensitive spectroscopic methods, other important mechanistic questions have eluded experimental determination. Here, we use computational quantum chemistry to refine the mechanism of the HydG catalytic reaction. We utilize quantum mechanics/molecular mechanics simulations to investigate the reactions at the canonical Fe–S cluster, where a radical cleavage of the tyrosine substrate takes place and proceeds through a relay of radical intermediates to form HCN and a COO•– radical anion. We then carry out a broken-symmetry density functional theory study of the reactions at the unusual five-iron auxiliary Fe–S cluster, where two equivalents of CN– and COOH• coordinate to the fifth “dangler iron” in a series of substitution and redox reactions that yield the synthon as the final product for further processing by HydE.
中文翻译:

氢气催化合成 Fe(II)(CO)2(CN) 半胱氨酸前体到 [FeFe] 氢化酶 H 簇的自由基中继机制的量子化学研究
[FeFe] 氢化酶催化质子和 H 2与 Fe-S“H-簇”的氧化还原相互转化,使用 CO、CN 和氮杂二硫醇配体到两个 Fe 中心。H 簇的生物合成是一个非常有趣的反应,由一组称为 HydE、HydF 和 HydG 的 Fe-S 成熟酶进行。HydG 是自由基S -腺苷甲硫氨酸 (rSAM) 家族的成员,可将酪氨酸、半胱氨酸和 Fe(II) 转化为有机金属 Fe(II)(CO) 2(CN) 半胱氨酸“合成子”,用作 HydE 的底物。尽管 HydG 机制的关键方面已通过同位素敏感光谱方法通过实验确定,但其他重要的机械问题仍未通过实验确定。在这里,我们使用计算量子化学来改进 HydG 催化反应的机制。我们利用量子力学/分子力学模拟来研究典型 Fe-S 簇的反应,其中酪氨酸底物发生自由基裂解,并通过自由基中间体的接力进行,形成 HCN 和 COO •-自由基阴离子。然后,我们对不寻常的五铁辅助 Fe-S 团簇的反应进行了对称性破缺密度泛函理论研究,其中两个当量的 CN -和 COOH •在一系列取代和氧化还原反应中与第五个“吊环铁”配位,产生合成子作为 HydE 进一步加工的最终产物。
更新日期:2021-10-12
中文翻译:

氢气催化合成 Fe(II)(CO)2(CN) 半胱氨酸前体到 [FeFe] 氢化酶 H 簇的自由基中继机制的量子化学研究
[FeFe] 氢化酶催化质子和 H 2与 Fe-S“H-簇”的氧化还原相互转化,使用 CO、CN 和氮杂二硫醇配体到两个 Fe 中心。H 簇的生物合成是一个非常有趣的反应,由一组称为 HydE、HydF 和 HydG 的 Fe-S 成熟酶进行。HydG 是自由基S -腺苷甲硫氨酸 (rSAM) 家族的成员,可将酪氨酸、半胱氨酸和 Fe(II) 转化为有机金属 Fe(II)(CO) 2(CN) 半胱氨酸“合成子”,用作 HydE 的底物。尽管 HydG 机制的关键方面已通过同位素敏感光谱方法通过实验确定,但其他重要的机械问题仍未通过实验确定。在这里,我们使用计算量子化学来改进 HydG 催化反应的机制。我们利用量子力学/分子力学模拟来研究典型 Fe-S 簇的反应,其中酪氨酸底物发生自由基裂解,并通过自由基中间体的接力进行,形成 HCN 和 COO •-自由基阴离子。然后,我们对不寻常的五铁辅助 Fe-S 团簇的反应进行了对称性破缺密度泛函理论研究,其中两个当量的 CN -和 COOH •在一系列取代和氧化还原反应中与第五个“吊环铁”配位,产生合成子作为 HydE 进一步加工的最终产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号