当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong Acids or Bases Displaced by Weak Acids or Bases in Aerosols: Reactions Driven by the Continuous Partitioning of Volatile Products into the Gas Phase
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.accounts.1c00318 Zhe Chen 1 , Pai Liu 1 , Yong Liu 2 , Yun-Hong Zhang 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.accounts.1c00318 Zhe Chen 1 , Pai Liu 1 , Yong Liu 2 , Yun-Hong Zhang 1
Affiliation
|
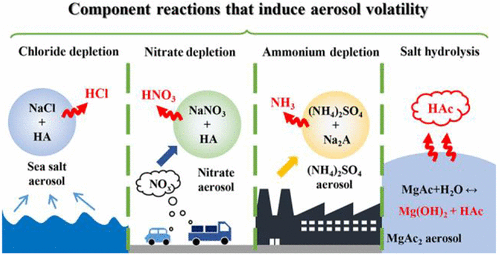
Aerosols are ubiquitous in the atmosphere and profoundly affect climate systems and human health. To gain more insights on their broad impacts, we need to comprehensively understand the fundamental properties of atmospheric aerosols. Since aerosols are multiphase, a dispersion of condensed matter (solid particles or liquid droplets, hereafter particles) in gas, partitioning of volatile matter between the condensed and the gas phases is one defining characteristic of aerosols. For example, water content partitioning under different relative humidity conditions, known as aerosol hygroscopicity, has been extensively investigated in the past decades. Meanwhile, partitioning of volatile organic or inorganic components, which is referred to as aerosol volatility, remains understudied. Commonly, a bulk solution system is treated as a single phase, with volatility mainly determined by the nature of its components, and the composition partitioning between solution and gas phase is limited. Aerosols, however, comprise an extensive gas phase, and their volatility can also be induced by component reactions. These reactions occurring within aerosols are driven by the formation of volatile products and their continuous partitioning into the gas phase. As a consequence, the overall aerosol systems exhibit prominent volatility. Noteworthily, such volatility induced by reactions is a phenomenon exclusively observed in the multiphase aerosol systems, and it is trivial in bulk solutions due to the limited extent of liquid–gas partitioning. Take the chloride depletion in sea salt particles as an example. Recent findings have revealed that chloride depletion can be caused by reactions between NaCl and weak organic acids, which release HCl into the gas phase. Such a reaction can be described as a strong acid displaced by a weak acid, which is hardly observed in bulk phase. Generally, this unique partitioning behavior of aerosol systems and its potential to alter aerosol composition, size, reactivity, and other physicochemical properties merits more attention by atmospheric community.
中文翻译:

强酸或强碱被气溶胶中的弱酸或碱置换:由挥发性产物连续分配到气相驱动的反应
气溶胶在大气中无处不在,对气候系统和人类健康有着深远的影响。为了更深入地了解它们的广泛影响,我们需要全面了解大气气溶胶的基本特性。由于气溶胶是多相的,凝聚物(固体颗粒或液滴,以下简称为粒子)在气体中的分散体,挥发性物质在凝聚态和气相之间的分配是气溶胶的一个定义特征。例如,不同相对湿度条件下的水含量分配,称为气溶胶吸湿性,在过去几十年中得到了广泛的研究。同时,挥发性有机或无机成分的分配(称为气溶胶挥发性)仍未得到充分研究。通常,整体溶液系统被视为单相,挥发性主要由其组分的性质决定,溶液和气相之间的组分分配是有限的。然而,气溶胶包含大量气相,并且它们的挥发性也可由组分反应引起。气溶胶中发生的这些反应是由挥发性产物的形成及其连续分配到气相中驱动的。因此,整个气溶胶系统表现出显着的波动性。值得注意的是,由反应引起的这种挥发性是多相气溶胶系统中独有的现象,并且由于液气分配的程度有限,它在散装溶液中是微不足道的。以海盐颗粒中的氯化物消耗为例。最近的研究结果表明,氯化钠耗尽可能是由 NaCl 和弱有机酸之间的反应引起的,后者将 HCl 释放到气相中。这种反应可以被描述为强酸被弱酸取代,这在体相中很难观察到。一般来说,气溶胶系统的这种独特的分配行为及其改变气溶胶成分、大小、反应性和其他物理化学性质的潜力值得大气界更多关注。
更新日期:2021-10-06
中文翻译:

强酸或强碱被气溶胶中的弱酸或碱置换:由挥发性产物连续分配到气相驱动的反应
气溶胶在大气中无处不在,对气候系统和人类健康有着深远的影响。为了更深入地了解它们的广泛影响,我们需要全面了解大气气溶胶的基本特性。由于气溶胶是多相的,凝聚物(固体颗粒或液滴,以下简称为粒子)在气体中的分散体,挥发性物质在凝聚态和气相之间的分配是气溶胶的一个定义特征。例如,不同相对湿度条件下的水含量分配,称为气溶胶吸湿性,在过去几十年中得到了广泛的研究。同时,挥发性有机或无机成分的分配(称为气溶胶挥发性)仍未得到充分研究。通常,整体溶液系统被视为单相,挥发性主要由其组分的性质决定,溶液和气相之间的组分分配是有限的。然而,气溶胶包含大量气相,并且它们的挥发性也可由组分反应引起。气溶胶中发生的这些反应是由挥发性产物的形成及其连续分配到气相中驱动的。因此,整个气溶胶系统表现出显着的波动性。值得注意的是,由反应引起的这种挥发性是多相气溶胶系统中独有的现象,并且由于液气分配的程度有限,它在散装溶液中是微不足道的。以海盐颗粒中的氯化物消耗为例。最近的研究结果表明,氯化钠耗尽可能是由 NaCl 和弱有机酸之间的反应引起的,后者将 HCl 释放到气相中。这种反应可以被描述为强酸被弱酸取代,这在体相中很难观察到。一般来说,气溶胶系统的这种独特的分配行为及其改变气溶胶成分、大小、反应性和其他物理化学性质的潜力值得大气界更多关注。











































 京公网安备 11010802027423号
京公网安备 11010802027423号