当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-sensitive dual-prodrug nanoparticles with light-controlled endo/lysosomal escape for synergistic photoactivated chemotherapy
Biomaterials Science ( IF 5.8 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1bm01154e Hongtong Lu 1, 2 , Shasha He 1 , Qingfei Zhang 1, 2 , Xiaoyuan Li 3 , Zhigang Xie 1, 2 , Zigui Wang 4 , Yanxin Qi 1 , Yubin Huang 1, 3
Biomaterials Science ( IF 5.8 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1bm01154e Hongtong Lu 1, 2 , Shasha He 1 , Qingfei Zhang 1, 2 , Xiaoyuan Li 3 , Zhigang Xie 1, 2 , Zigui Wang 4 , Yanxin Qi 1 , Yubin Huang 1, 3
Affiliation
|
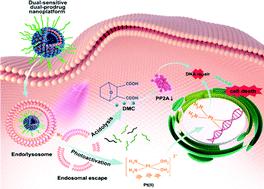
The clinical application of conventional chemotherapeutic agents, represented by cisplatin, is limited by severe side effects. So, it is essential to explore more safer and controlled drug delivery systems for synergistic chemotherapy. In this work, we designed dual-sensitive dual-prodrug nanoparticles (DDNPs) for photoactivated platinum-based synergistic chemotherapy. With photosensitivity, DDNPs could be photoactivated from inert Pt(IV) to toxic Pt(II) under safe UVA light in a spatiotemporally controlled manner. Concurrently, mild 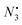 could be generated from DDNPs to assist the endo/lysosomal escape of DDNPs for better photoactivated chemotherapy (PACT). Furthermore, with acid-sensitivity, demethylcantharidin (DMC), a protein phosphatase 2A (PP2A) inhibitor, was released to block the DNA repair pathway and thereby could sensitize platinum-based chemotherapy in intracellular acidic microenvironments. Along with a precise ratio (Pt : DMC = 1 : 2), DDNPs had a powerful synergistic anti-cancer effect in vitro and in vivo. In the future, DDNPs have great potential as a safe and multifunctional drug delivery system for precise nanomedicine in clinical treatments.
could be generated from DDNPs to assist the endo/lysosomal escape of DDNPs for better photoactivated chemotherapy (PACT). Furthermore, with acid-sensitivity, demethylcantharidin (DMC), a protein phosphatase 2A (PP2A) inhibitor, was released to block the DNA repair pathway and thereby could sensitize platinum-based chemotherapy in intracellular acidic microenvironments. Along with a precise ratio (Pt : DMC = 1 : 2), DDNPs had a powerful synergistic anti-cancer effect in vitro and in vivo. In the future, DDNPs have great potential as a safe and multifunctional drug delivery system for precise nanomedicine in clinical treatments.
中文翻译:

具有光控制内/溶酶体逃逸的双敏感双前药纳米粒子,用于协同光活化化疗
以顺铂为代表的常规化疗药物的临床应用受到严重副作用的限制。因此,探索更安全和可控的协同化疗药物输送系统至关重要。在这项工作中,我们设计了用于光活化铂基协同化疗的双敏感双前药纳米粒子 (DDNPs)。由于具有光敏性,DDNPs 可以在安全的 UVA 光下以时空可控的方式从惰性 Pt( IV )光活化为有毒 Pt( II )。同时,温和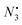 可以从 DDNPs 产生,以帮助 DDNPs 的内/溶酶体逃逸,以获得更好的光活化化疗 (PACT)。此外,由于具有酸敏感性,脱甲基斑蝥素 (DMC),一种蛋白磷酸酶 2A (PP2A) 抑制剂被释放以阻断 DNA 修复途径,从而可以在细胞内酸性微环境中使铂类化疗敏感。除了精确的比例(Pt : DMC = 1 : 2)外,DDNPs在体外和体内具有强大的协同抗癌作用。未来,DDNPs 作为一种安全、多功能的药物递送系统,在临床治疗中用于精准的纳米医学具有巨大的潜力。
可以从 DDNPs 产生,以帮助 DDNPs 的内/溶酶体逃逸,以获得更好的光活化化疗 (PACT)。此外,由于具有酸敏感性,脱甲基斑蝥素 (DMC),一种蛋白磷酸酶 2A (PP2A) 抑制剂被释放以阻断 DNA 修复途径,从而可以在细胞内酸性微环境中使铂类化疗敏感。除了精确的比例(Pt : DMC = 1 : 2)外,DDNPs在体外和体内具有强大的协同抗癌作用。未来,DDNPs 作为一种安全、多功能的药物递送系统,在临床治疗中用于精准的纳米医学具有巨大的潜力。
更新日期:2021-09-27
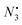 could be generated from DDNPs to assist the endo/lysosomal escape of DDNPs for better photoactivated chemotherapy (PACT). Furthermore, with acid-sensitivity, demethylcantharidin (DMC), a protein phosphatase 2A (PP2A) inhibitor, was released to block the DNA repair pathway and thereby could sensitize platinum-based chemotherapy in intracellular acidic microenvironments. Along with a precise ratio (Pt : DMC = 1 : 2), DDNPs had a powerful synergistic anti-cancer effect in vitro and in vivo. In the future, DDNPs have great potential as a safe and multifunctional drug delivery system for precise nanomedicine in clinical treatments.
could be generated from DDNPs to assist the endo/lysosomal escape of DDNPs for better photoactivated chemotherapy (PACT). Furthermore, with acid-sensitivity, demethylcantharidin (DMC), a protein phosphatase 2A (PP2A) inhibitor, was released to block the DNA repair pathway and thereby could sensitize platinum-based chemotherapy in intracellular acidic microenvironments. Along with a precise ratio (Pt : DMC = 1 : 2), DDNPs had a powerful synergistic anti-cancer effect in vitro and in vivo. In the future, DDNPs have great potential as a safe and multifunctional drug delivery system for precise nanomedicine in clinical treatments.
中文翻译:

具有光控制内/溶酶体逃逸的双敏感双前药纳米粒子,用于协同光活化化疗
以顺铂为代表的常规化疗药物的临床应用受到严重副作用的限制。因此,探索更安全和可控的协同化疗药物输送系统至关重要。在这项工作中,我们设计了用于光活化铂基协同化疗的双敏感双前药纳米粒子 (DDNPs)。由于具有光敏性,DDNPs 可以在安全的 UVA 光下以时空可控的方式从惰性 Pt( IV )光活化为有毒 Pt( II )。同时,温和
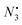 可以从 DDNPs 产生,以帮助 DDNPs 的内/溶酶体逃逸,以获得更好的光活化化疗 (PACT)。此外,由于具有酸敏感性,脱甲基斑蝥素 (DMC),一种蛋白磷酸酶 2A (PP2A) 抑制剂被释放以阻断 DNA 修复途径,从而可以在细胞内酸性微环境中使铂类化疗敏感。除了精确的比例(Pt : DMC = 1 : 2)外,DDNPs在体外和体内具有强大的协同抗癌作用。未来,DDNPs 作为一种安全、多功能的药物递送系统,在临床治疗中用于精准的纳米医学具有巨大的潜力。
可以从 DDNPs 产生,以帮助 DDNPs 的内/溶酶体逃逸,以获得更好的光活化化疗 (PACT)。此外,由于具有酸敏感性,脱甲基斑蝥素 (DMC),一种蛋白磷酸酶 2A (PP2A) 抑制剂被释放以阻断 DNA 修复途径,从而可以在细胞内酸性微环境中使铂类化疗敏感。除了精确的比例(Pt : DMC = 1 : 2)外,DDNPs在体外和体内具有强大的协同抗癌作用。未来,DDNPs 作为一种安全、多功能的药物递送系统,在临床治疗中用于精准的纳米医学具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号