Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-09-25 , DOI: 10.1016/j.colsurfa.2021.127629 Ahlem Eddehech 1, 2 , Nabil Smichi 3 , Sebastien Violot 4 , Emmanuel Bettler 5 , Leyre Brizuela 6 , Alexandre Noiriel 2 , Abdelkarim Abousalham 2 , Zied Zarai 1, 7
|
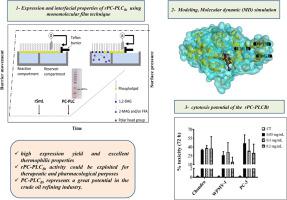
A novel alkaline thermostable phosphatidylcholine-specific phospholipase C (PC-PLCBt) was expressed in E. coli system. Recombinant PC-PLCBt (rPC-PLCBt) activity and thermostability were shown to be significantly dependent on the Zn2+. The maximum rPC-PLCBt catalytic activity was found to be 1372 U mg-1 in the presence of 0.1 mM Zn2+ and at 60 °C using an Egg PC as substrate. The interfacial kinetic data show that nPC-PLCBt and rPC-PLCBt display similar substrate specificity on various phospholipid monolayers. The maximal rPC-PLCBt activities were recorded, at decreasing order, on 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), 1,2-dilauroyl-sn-glycero-3-phosphoethanolamine (DLPE), 1,2-diacyl-sn-phosphoglycerol (PG), and 1,2-diacyl-sn-phosphoserine (PS) monolayers at interfacial surface pressures of 15, 25, 20, and 25 mN m-1, respectively. Such important penetrating power could be exploited for pharmacological purposes. The highest activities were recorded on the DLPC monolayer and shown to be 121.61 and 40.13 mmol cm-2 min-1 M-1 for native and recombinant PC-PLCBt, respectively. Interestingly, compared to all known Bacillus PLCs, both PC-PLCBt forms showed an exclusive capacity to hydrolyze the PG film with a more pronounced rate of hydrolysis for the native form with a specific activity of 58.29 mmol cm-2 min-1 M-1. Therefore, the high enzyme level production of about 14 mg L-1, the thermostability as well as the broad phospholipid specificity of PC-PLCBt represent great potential in the crude oil refining industry.
中文翻译:

破译来自苏云金芽孢杆菌的重组热稳定磷脂酰胆碱特异性磷脂酶 C 活性:生化和界面特性
在大肠杆菌系统中表达了一种新型碱性热稳定磷脂酰胆碱特异性磷脂酶 C (PC-PLC Bt ) 。重组PC-PLC Bt (rPC-PLC Bt ) 活性和热稳定性显示出显着依赖于Zn 2+。发现最大 rPC-PLC Bt催化活性在 0.1 mM Zn 2+存在下和在 60 °C 下使用 Egg PC 作为底物为 1372 U mg -1。界面动力学数据表明,nPC-PLC Bt和 rPC-PLC Bt在各种磷脂单层上显示出相似的底物特异性。最大 rPC-PLC Bt以降序记录 1,2-二月桂酰-sn-甘油-3-磷酸胆碱 (DLPC)、1,2-二月桂酰-sn-甘油-3-磷酸乙醇胺 (DLPE)、1,2-二酰基-sn的活性-磷酸甘油(PG) 和 1,2-二酰基-sn - 磷酸丝氨酸(PS) 单层,界面压力分别为15、25、20和 25 mN m -1。这种重要的穿透力可以用于药理学目的。最高活性记录在 DLPC 单层上,对于天然和重组 PC-PLC Bt分别显示为 121.61 和 40.13 mmol cm -2 min -1 M -1。有趣的是,与所有已知的相比芽孢杆菌PLC,两种 PC-PLC Bt形式都显示出独特的水解 PG 膜的能力,天然形式的水解速率更显着,比活性为 58.29 mmol cm -2 min -1 M -1。因此,PC-PLC Bt的约 14 mg L -1的高酶水平产量、热稳定性以及广泛的磷脂特异性在原油精炼工业中具有巨大的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号