Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-09-26 , DOI: 10.1016/j.jhazmat.2021.127358 Rieks de Rink 1 , Micaela B Lavender 2 , Dandan Liu 3 , Johannes B M Klok 4 , Dimitry Y Sorokin 5 , Annemiek Ter Heijne 2 , Cees J N Buisman 6
|
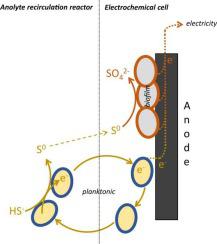
Sulfide oxidizing bacteria (SOB) are widely applied in industry to convert toxic H2S into elemental sulfur. Haloalkaliphilic planktonic SOB can remove sulfide from solution under anaerobic conditions (SOB are ‘charged’), and release electrons at an electrode (discharge of SOB). The effect of this electron shuttling on product formation and biomass growth is not known. Here, we study and demonstrate a continuous process in which SOB remove sulfide from solution in an anaerobic ‘uptake chamber’, and shuttle these electrons to the anode of an electrochemical cell, in the absence of dissolved sulfide. Two experiments over 31 and 41 days were performed. At a sulfide loading rate of 1.1 mmolS/day, electricity was produced continuously (3 A/m2) without dissolved sulfide in the anolyte. The main end product was sulfate (56% in experiment 1 and 78% in experiment 2), and 87% and 77% of the electrons in sulfide were recovered as electricity. It was found that the current density was dependent on the sulfide loading rate and not on the anode potential. Biological growth occurred, mainly at the anode as biofilm, in which the deltaproteobacterial genus Desulfurivibrio was dominating. Our results demonstrate a novel strategy to produce electricity from sulfide in an electrochemical system.
中文翻译:

硫化物氧化细菌的连续电子穿梭作为一种产生电流的新策略
硫化物氧化菌 (SOB) 广泛应用于工业中将有毒的 H 2 S 转化为元素硫。嗜卤碱浮游 SOB 可以在厌氧条件下从溶液中去除硫化物(SOB 是“带电的”),并在电极上释放电子(SOB 放电)。这种电子穿梭对产物形成和生物质生长的影响尚不清楚。在这里,我们研究并展示了一个连续过程,其中 SOB 在厌氧“吸收室”中从溶液中去除硫化物,并在没有溶解硫化物的情况下将这些电子传送到电化学电池的阳极。进行了 31 天和 41 天的两个实验。在 1.1 mmolS/天的硫化物负载率下,连续发电(3 A/m 2) 在阳极电解液中不溶解硫化物。主要最终产物是硫酸盐(实验 1 中为 56%,实验 2 中为 78%),硫化物中 87% 和 77% 的电子被回收为电能。发现电流密度取决于硫化物负载率而不是阳极电位。发生生物生长,主要在阳极作为生物膜,其中 deltaproteobacterial 属 Desulfurivibrio占主导地位。我们的研究结果证明了一种在电化学系统中从硫化物发电的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号