当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Naturally Occurring Mutations to Muscle-Type Creatine Kinase Impact Its Canonical and Pharmacological Activities in a Substrate-Dependent Manner In Vitro
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-12-01 , DOI: 10.1124/molpharm.121.000348 Eric P Mosher 1 , Colten D Eberhard 1 , Namandjé N Bumpus 2
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-12-01 , DOI: 10.1124/molpharm.121.000348 Eric P Mosher 1 , Colten D Eberhard 1 , Namandjé N Bumpus 2
Affiliation
|
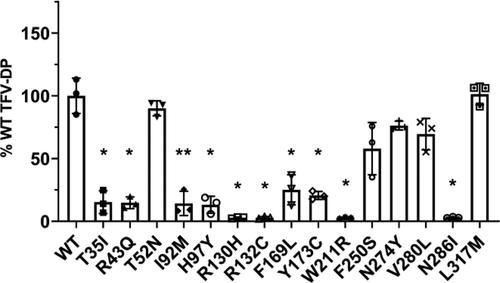
Tenofovir (TFV) is a key component of human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP). TFV is a nucleotide analog reverse-transcriptase inhibitor prodrug that requires two separate phosphorylation reactions by intracellular kinases to form the active metabolite tenofovir-diphosphate (TFV-DP). Muscle-type creatine kinase (CKM) has previously been demonstrated to be the kinase most responsible for the phosphorylation of tenofovir-monophosphate (TFV-MP) to the active metabolite in colon tissue. Because of the importance of CKM in TFV activation, genetic variation in CKM may contribute to interindividual variability in TFV-DP levels. In the present study, we report 10 naturally occurring CKM mutations that reduced TFV-MP phosphorylation in vitro: T35I, R43Q, I92M, H97Y, R130H, R132C, F169L, Y173C, W211R, V280L, and N286I. Interestingly, of these 10, only 4—R130H, R132C, W211R, and N286I—reduced both canonical CKM activities: ADP phosphorylation and ATP dephosphorylation. Although positions 130, 132, and 286 are located in the active site, the other mutations that resulted in decreased TFV-MP phosphorylation occur elsewhere in the protein structure. Four of these eight mutations—T35I, R43Q, I92M, and W211R—were found to decrease the thermal stability of the protein. Additionally, the W211R mutation was found to impact protein structure both locally and at a distance. These data suggest a substrate-specific effect such that certain mutations are tolerated for canonical activities while being deleterious toward the pharmacological activity of TFV activation, which could influence PrEP outcomes.
中文翻译:

肌肉型肌酸激酶自然发生的突变在体外以底物依赖性方式影响其典型和药理活性
替诺福韦 (TFV) 是人类免疫缺陷病毒 (HIV) 暴露前预防 (PrEP) 的关键成分。 TFV 是一种核苷酸类似物逆转录酶抑制剂前药,需要细胞内激酶进行两次单独的磷酸化反应才能形成活性代谢物替诺福韦二磷酸 (TFV-DP)。肌肉型肌酸激酶 (CKM) 先前已被证明是对替诺福韦单磷酸 (TFV-MP) 磷酸化为结肠组织中活性代谢物最重要的激酶。由于 CKM 在 TFV 激活中的重要性,CKM 的遗传变异可能导致 TFV-DP 水平的个体差异。在本研究中,我们报告了 10 种自然发生的 CKM 突变,可在体外降低 TFV-MP 磷酸化:T35I、R43Q、I92M、H97Y、R130H、R132C、F169L、Y173C、W211R、V280L 和 N286I。有趣的是,在这 10 个中,只有 4 个(R130H、R132C、W211R 和 N286I)降低了两种典型的 CKM 活性:ADP 磷酸化和 ATP 去磷酸化。尽管位置 130、132 和 286 位于活性位点,但导致 TFV-MP 磷酸化降低的其他突变发生在蛋白质结构的其他位置。这八个突变中的四个——T35I、R43Q、I92M 和 W211R——被发现会降低蛋白质的热稳定性。此外,还发现 W211R 突变会影响局部和远距离的蛋白质结构。这些数据表明底物特异性效应,使得某些突变对于典型活性是耐受的,同时对 TFV 激活的药理活性有害,这可能会影响 PrEP 结果。
更新日期:2021-11-20
中文翻译:

肌肉型肌酸激酶自然发生的突变在体外以底物依赖性方式影响其典型和药理活性
替诺福韦 (TFV) 是人类免疫缺陷病毒 (HIV) 暴露前预防 (PrEP) 的关键成分。 TFV 是一种核苷酸类似物逆转录酶抑制剂前药,需要细胞内激酶进行两次单独的磷酸化反应才能形成活性代谢物替诺福韦二磷酸 (TFV-DP)。肌肉型肌酸激酶 (CKM) 先前已被证明是对替诺福韦单磷酸 (TFV-MP) 磷酸化为结肠组织中活性代谢物最重要的激酶。由于 CKM 在 TFV 激活中的重要性,CKM 的遗传变异可能导致 TFV-DP 水平的个体差异。在本研究中,我们报告了 10 种自然发生的 CKM 突变,可在体外降低 TFV-MP 磷酸化:T35I、R43Q、I92M、H97Y、R130H、R132C、F169L、Y173C、W211R、V280L 和 N286I。有趣的是,在这 10 个中,只有 4 个(R130H、R132C、W211R 和 N286I)降低了两种典型的 CKM 活性:ADP 磷酸化和 ATP 去磷酸化。尽管位置 130、132 和 286 位于活性位点,但导致 TFV-MP 磷酸化降低的其他突变发生在蛋白质结构的其他位置。这八个突变中的四个——T35I、R43Q、I92M 和 W211R——被发现会降低蛋白质的热稳定性。此外,还发现 W211R 突变会影响局部和远距离的蛋白质结构。这些数据表明底物特异性效应,使得某些突变对于典型活性是耐受的,同时对 TFV 激活的药理活性有害,这可能会影响 PrEP 结果。










































 京公网安备 11010802027423号
京公网安备 11010802027423号