当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Expression and Characterization of the Highly Promiscuous Lanthipeptide Synthetase SyncM, Enabling the Production of Lanthipeptides with a Broad Range of Ring Topologies
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acssynbio.1c00224 Patricia Arias-Orozco 1 , Maartje Inklaar 1 , Judith Lanooij 1 , Rubén Cebrián 1 , Oscar P Kuipers 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acssynbio.1c00224 Patricia Arias-Orozco 1 , Maartje Inklaar 1 , Judith Lanooij 1 , Rubén Cebrián 1 , Oscar P Kuipers 1
Affiliation
|
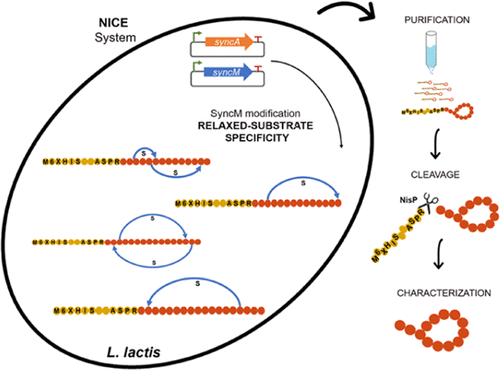
Lanthipeptides are ribosomally synthesized and post-translationally modified peptides characterized by the presence of lanthionine rings that provide stability and functionality. Genome mining techniques have shown their huge diversity and potential for the discovery of novel active molecules. However, in many cases, they are not easily produced under laboratory conditions. The heterologous expression of these molecules using well-characterized lanthipeptide biosynthetic enzymes is rising as an alternative system for the design and production of new lanthipeptides with biotechnological or clinical properties. Nevertheless, the substrate-enzyme specificity limits the complete modification of the desired peptides and hence, their full stability and/or biological activity. New low substrate-selective biosynthetic enzymes are therefore necessary for the heterologous production of new-to-nature peptides. Here, we have identified, cloned, and heterologously expressed in Lactococcus lactis the most promiscuous lanthipeptide synthetase described to date, i.e., SyncM from the marine cyanobacteria Synechococcus MITS9509. We have characterized the functionality of SyncM by the successful expression of 15 out of 18 different SyncA substrates, subsequently determining the dehydration and cyclization processes in six representatives of them. This characterization highlights the very relaxed substrate specificity of SyncM toward its precursors and the ability to catalyze the formation of exceptionally large rings in a variety of topologies. Our results suggest that SyncM could be an attractive enzyme to design and produce a wide variety of new-to-nature lanthipeptides with a broad range of ring topologies.
中文翻译:

高度混杂的羊毛肽合成酶 SyncM 的功能表达和表征,使生产具有广泛环拓扑结构的羊毛肽成为可能
羊毛硫肽是核糖体合成和翻译后修饰的肽,其特征在于存在提供稳定性和功能性的羊毛硫氨酸环。基因组挖掘技术已显示出其巨大的多样性和发现新活性分子的潜力。然而,在许多情况下,它们在实验室条件下不容易生产。使用充分表征的羊毛肽生物合成酶的这些分子的异源表达正在上升为用于设计和生产具有生物技术或临床特性的新羊毛肽的替代系统。然而,底物-酶特异性限制了所需肽的完全修饰,因此限制了它们的完全稳定性和/或生物活性。因此,新的低底物选择性生物合成酶对于异源生产新的天然肽是必要的。在这里,我们已经在乳酸乳球菌是迄今为止描述的最混杂的羊毛肽合成酶,即来自海洋蓝细菌Synechococcus MITS9509 的SyncM。我们通过成功表达 18 种不同 SyncA 底物中的 15 种来表征 SyncM 的功能,随后确定其中 6 种代表的脱水和环化过程。这种表征突出了 SyncM 对其前体的非常宽松的底物特异性以及在各种拓扑结构中催化形成异常大环的能力。我们的研究结果表明,SyncM 可能是一种有吸引力的酶,可用于设计和生产各种具有广泛环拓扑结构的新型天然羊毛肽。
更新日期:2021-10-15
中文翻译:

高度混杂的羊毛肽合成酶 SyncM 的功能表达和表征,使生产具有广泛环拓扑结构的羊毛肽成为可能
羊毛硫肽是核糖体合成和翻译后修饰的肽,其特征在于存在提供稳定性和功能性的羊毛硫氨酸环。基因组挖掘技术已显示出其巨大的多样性和发现新活性分子的潜力。然而,在许多情况下,它们在实验室条件下不容易生产。使用充分表征的羊毛肽生物合成酶的这些分子的异源表达正在上升为用于设计和生产具有生物技术或临床特性的新羊毛肽的替代系统。然而,底物-酶特异性限制了所需肽的完全修饰,因此限制了它们的完全稳定性和/或生物活性。因此,新的低底物选择性生物合成酶对于异源生产新的天然肽是必要的。在这里,我们已经在乳酸乳球菌是迄今为止描述的最混杂的羊毛肽合成酶,即来自海洋蓝细菌Synechococcus MITS9509 的SyncM。我们通过成功表达 18 种不同 SyncA 底物中的 15 种来表征 SyncM 的功能,随后确定其中 6 种代表的脱水和环化过程。这种表征突出了 SyncM 对其前体的非常宽松的底物特异性以及在各种拓扑结构中催化形成异常大环的能力。我们的研究结果表明,SyncM 可能是一种有吸引力的酶,可用于设计和生产各种具有广泛环拓扑结构的新型天然羊毛肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号