当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Guaiacol Hydrogenation in Methanesulfonic Acid Using a Stirred Slurry Electrocatalytic Reactor: Mass Transport and Reaction Kinetics Aspects
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-23 , DOI: 10.1021/acssuschemeng.1c03332 Yanuar Philip Wijaya 1, 2 , Robertus D. D. Putra 1, 2 , Kevin J. Smith 1 , Chang Soo Kim 2, 3 , Elöd L. Gyenge 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-23 , DOI: 10.1021/acssuschemeng.1c03332 Yanuar Philip Wijaya 1, 2 , Robertus D. D. Putra 1, 2 , Kevin J. Smith 1 , Chang Soo Kim 2, 3 , Elöd L. Gyenge 1
Affiliation
|
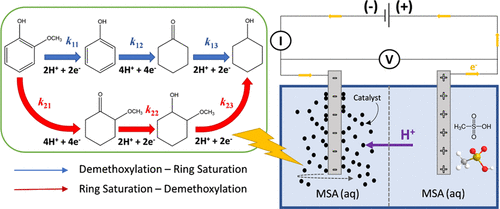
The electrocatalytic reduction of guaiacol, a lignin model compound, is investigated in a stirred slurry electrocatalytic reactor (SSER) under mild conditions (1 atm, 30–60 °C). Methanesulfonic acid (MSA) is used as an electrolyte due to its eco-friendly properties along with comparable ionic conductivity to the mineral acids (e.g., sulfuric acid and perchloric acid). Mass transport and kinetic aspects are investigated combining the experimental results obtained under either galvanostatic or potentiostatic control with reaction network kinetic models. The reactant mass transport rate to the catalyst particle surface, together with the collision rates among catalyst particles and the current collector, respectively, has a significant effect on guaiacol conversion and Faradaic efficiency. Therefore, optimum stirring is necessary to ensure good electric contact in the catalyst bed slurry to achieve substantial electrocatalytic hydrogenation (ECH) reaction rates. In the absence of mass transfer limitation, reaction network kinetic modeling based on the Langmuir–Hinshelwood mechanism was performed and validated by the experimental data. Rate constants and activation energies were calculated, and it was found that phenol hydrogenation was the fastest reaction, while 2-methoxycyclohexanol demethoxylation was the slowest in the overall guaiacol ECH network. Furthermore, we show that the SSER can be operated at industrially relevant cathode superficial current densities (>100 mA cm–2), thereby, opening new and practical possibilities for the sustainable valorization of biomass-derived compounds.
中文翻译:

使用搅拌浆液电催化反应器在甲磺酸中加氢愈创木酚:传质和反应动力学方面
在温和条件(1 atm,30-60°C)下,在搅拌浆液电催化反应器(SSER)中研究愈创木酚(一种木质素模型化合物)的电催化还原。甲磺酸 (MSA) 因其环保特性以及与无机酸(例如硫酸和高氯酸)相当的离子电导率而被用作电解质。将在恒电流或恒电位控制下获得的实验结果与反应网络动力学模型相结合,对传质和动力学方面进行了研究。反应物向催化剂颗粒表面的传质速率,以及催化剂颗粒和集电器之间的碰撞速率,分别对愈创木酚转化率和法拉第效率有显着影响。所以,最佳搅拌对于确保催化剂床浆液中的良好电接触以实现显着的电催化加氢 (ECH) 反应速率是必要的。在没有传质限制的情况下,基于 Langmuir-Hinshelwood 机制的反应网络动力学模型被执行并通过实验数据进行验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm 进行了基于 Langmuir-Hinshelwood 机制的反应网络动力学建模,并通过实验数据进行了验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm 进行了基于 Langmuir-Hinshelwood 机制的反应网络动力学建模,并通过实验数据进行了验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm–2 ),从而为生物质衍生化合物的可持续增值开辟了新的实用可能性。
更新日期:2021-10-04
中文翻译:

使用搅拌浆液电催化反应器在甲磺酸中加氢愈创木酚:传质和反应动力学方面
在温和条件(1 atm,30-60°C)下,在搅拌浆液电催化反应器(SSER)中研究愈创木酚(一种木质素模型化合物)的电催化还原。甲磺酸 (MSA) 因其环保特性以及与无机酸(例如硫酸和高氯酸)相当的离子电导率而被用作电解质。将在恒电流或恒电位控制下获得的实验结果与反应网络动力学模型相结合,对传质和动力学方面进行了研究。反应物向催化剂颗粒表面的传质速率,以及催化剂颗粒和集电器之间的碰撞速率,分别对愈创木酚转化率和法拉第效率有显着影响。所以,最佳搅拌对于确保催化剂床浆液中的良好电接触以实现显着的电催化加氢 (ECH) 反应速率是必要的。在没有传质限制的情况下,基于 Langmuir-Hinshelwood 机制的反应网络动力学模型被执行并通过实验数据进行验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm 进行了基于 Langmuir-Hinshelwood 机制的反应网络动力学建模,并通过实验数据进行了验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm 进行了基于 Langmuir-Hinshelwood 机制的反应网络动力学建模,并通过实验数据进行了验证。计算了速率常数和活化能,发现在整个愈创木酚 ECH 网络中,苯酚加氢反应最快,而 2-甲氧基环己醇脱甲氧基反应最慢。此外,我们表明 SSER 可以在工业相关的阴极表面电流密度 (>100 mA cm–2 ),从而为生物质衍生化合物的可持续增值开辟了新的实用可能性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号