Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microvessel-on-Chip Fabrication for the In Vitro Modeling of Nanomedicine Transport
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c00735 Sergio Dávila 1 , Jean Cacheux 1 , Isabel Rodríguez 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c00735 Sergio Dávila 1 , Jean Cacheux 1 , Isabel Rodríguez 1
Affiliation
|
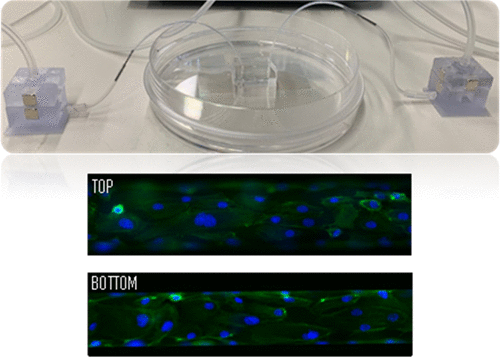
Tumor-on-chip devices are becoming ideal platforms to recreate in vitro the particular physiological microenvironment of interest for onco-nanomedicine testing and development. This work presents a strategy to produce a round artificial microvessel on-a-chip device for the study of physiologically relevant nanomedicine transport dynamics. The microchannels have a diameter in the range of the tumor capillaries and a semicircular geometry. This geometry is obtained through an intermediate thermal nanoimprint step using a master mold with square-shaped channel structures produced by standard silicon micromachining or by stereolithography three-dimensional (3D) printing. The working microfluidic chip devices are made by casting polydimethylsiloxane on the imprinted intermediate mold. Artificial blood microvessels are created by seeding human endothelial cells into the round-shaped channels acting as the scaffold. The microchip is connected by 3D-printed reservoirs to a pressure controller, allowing for a fine fluidic control. Under physiological flow conditions, the dynamic interaction of nanoparticles (NPs) with the artificial endothelium was assessed by high-magnification fluorescence microscopy. Overtime, internalization of NPs and clustering was observed and the accumulation rate into the endothelial cells could be characterized in real time.
中文翻译:

用于纳米药物传输体外建模的微血管芯片制造
片上肿瘤设备正在成为体外再造的理想平台对肿瘤纳米医学测试和开发感兴趣的特定生理微环境。这项工作提出了一种生产圆形人工微血管片上装置的策略,用于研究生理相关的纳米药物传输动力学。微通道具有肿瘤毛细血管范围内的直径和半圆形几何形状。这种几何形状是通过中间热纳米压印步骤获得的,该步骤使用具有方形通道结构的母模,由标准硅微加工或立体光刻三维 (3D) 打印产生。工作微流控芯片装置是通过在印迹中间模具上浇铸聚二甲基硅氧烷制成的。人造血液微血管是通过将人类内皮细胞接种到充当支架的圆形通道中来创建的。微芯片通过 3D 打印的储液器连接到压力控制器,从而实现精细的流体控制。在生理流动条件下,纳米颗粒 (NPs) 与人工内皮的动态相互作用通过高倍荧光显微镜进行评估。随着时间的推移,观察到 NPs 的内化和聚集,并且可以实时表征内皮细胞的积累率。
更新日期:2021-10-06
中文翻译:

用于纳米药物传输体外建模的微血管芯片制造
片上肿瘤设备正在成为体外再造的理想平台对肿瘤纳米医学测试和开发感兴趣的特定生理微环境。这项工作提出了一种生产圆形人工微血管片上装置的策略,用于研究生理相关的纳米药物传输动力学。微通道具有肿瘤毛细血管范围内的直径和半圆形几何形状。这种几何形状是通过中间热纳米压印步骤获得的,该步骤使用具有方形通道结构的母模,由标准硅微加工或立体光刻三维 (3D) 打印产生。工作微流控芯片装置是通过在印迹中间模具上浇铸聚二甲基硅氧烷制成的。人造血液微血管是通过将人类内皮细胞接种到充当支架的圆形通道中来创建的。微芯片通过 3D 打印的储液器连接到压力控制器,从而实现精细的流体控制。在生理流动条件下,纳米颗粒 (NPs) 与人工内皮的动态相互作用通过高倍荧光显微镜进行评估。随着时间的推移,观察到 NPs 的内化和聚集,并且可以实时表征内皮细胞的积累率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号