Energy Conversion and Management ( IF 9.9 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.enconman.2021.114761 Maria Anna Murmura 1 , Giorgio Vilardi 1
|
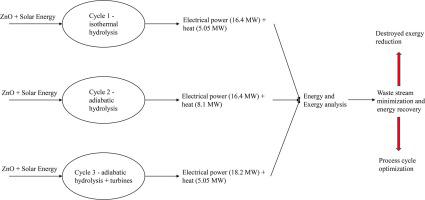
Water-splitting thermochemical cycles have emerged as a possible solution for the decentralized production of hydrogen using solar energy. A useful tool to select the most promising thermochemical cycles among those proposed in the literature is represented by the exergy analysis. In this context, the exergy and energy analyses of the zinc/zinc oxide thermochemical water-splitting cycle, coupled with the production of electrical energy in a fuel cell, are here carried out and discussed. The aim has been to identify the steps in the process characterized by the highest inefficiencies and to propose possible solutions to increase the overall thermodynamic efficiency. Calculations were carried out using the commercial process simulator PRO/II. The cycle consists of two steps: (i) the endothermic thermal reduction of solid zinc oxide to liquid zinc and oxygen, carried out in a solar thermo-reactor at 2025 °C, and (ii) the non-solar, exothermic hydrolysis of zinc to form hydrogen and solid zinc oxide. This work reports three possible process schemes based on the zinc/zinc oxide redox reactions, each differing from the others in terms of envisaged modes of heat recovery. In addition, the effect of carrying out the hydrolysis step under adiabatic, rather than isothermal conditions, has been analysed. Four values of conversion were considered (1, 0.9. 0.8, and 0.7) and the effect on fuel cell power generation, utility consumption, and energy and exergy efficiency was evaluated. It was found that the exergy efficiency increased from about 14% for the classical zinc/zinc oxide cycle to almost 40% in the most efficient scenario considered here. The variation of hydrogen conversion in the fuel cell led to a decrease in generated power, from 16.41 MW at hydrogen conversion of 1 down to 11.48 MW at hydrogen conversion of 0.7.
中文翻译:

用于制氢和燃料电池发电的锌/氧化锌热化学循环的能量和火用分析
水分解热化学循环已成为利用太阳能分散生产氢气的可能解决方案。火用分析代表了在文献中提出的那些中选择最有希望的热化学循环的有用工具。在此背景下,此处进行和讨论了锌/氧化锌热化学水分解循环的火用和能量分析,以及在燃料电池中产生电能。目的是确定过程中效率最高的步骤,并提出可能的解决方案以提高整体热力学效率。使用商业过程模拟器 PRO/II 进行计算。该循环包括两个步骤:(i) 在 2025 °C 的太阳能热反应器中进行的固体氧化锌吸热热还原为液体锌和氧气,以及 (ii) 锌的非太阳能放热水解形成氢气和固体氧化锌. 这项工作报告了基于锌/氧化锌氧化还原反应的三种可能的工艺方案,每个方案在热回收的设想模式方面都与其他方案不同。此外,还分析了在绝热而非等温条件下进行水解步骤的影响。考虑了四个转换值(1、0.9、0.8 和 0.7),并评估了对燃料电池发电、公用事业消耗以及能源和火用效率的影响。发现火用效率从经典锌/氧化锌循环的约 14% 增加到此处考虑的最有效场景中的近 40%。燃料电池中氢转化率的变化导致产生的功率下降,从氢转化率 1 时的 16.41 MW 下降到氢转化率 0.7 时的 11.48 MW。











































 京公网安备 11010802027423号
京公网安备 11010802027423号