Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-09-24 , DOI: 10.1016/j.colsurfa.2021.127622 Goknur Kara 1, 2, 3 , Soheil Malekghasemi 4 , Bulent Ozpolat 2, 5 , Emir Baki Denkbas 6
|
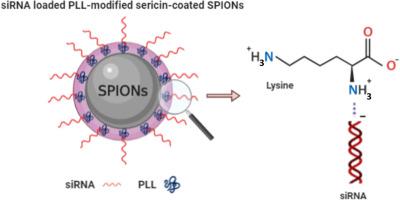
Small interfering RNA (siRNA) is a promising therapeutic modality, however, its successful clinical application is still challenging due to the lack of safe and efficient carrier systems. Superparamagnetic iron oxide nanoparticles (SPIONs)-based drug or gene carrier systems have displayed tremendous promise in nanomedicine. They possess intrinsic unique superparamagnetism that provides them to be concentrated in the targeted therapeutic site of action where an external magnetic field is applied. SPIONs can be also designed as theranostic agents to achieve simultaneous therapeutic and diagnostic purposes. Despite these favorable features, SPIONs are colloidally unstable and can be easily eliminated in the circulation. More importantly, the toxicological concerns associated with SPIONs, which often lead to the generation of reactive oxygen species (ROS), need to be thoroughly considered. Various types of polymers have been proposed so far to cover the surface of SPION to overcome these disadvantages. Silk protein, sericin can be ideal as a coating material due to its high biocompatibility, good biodegradability, and in vivo stability. In terms of the development of SPIONs as siRNA carriers, to the best of our knowledge, no protein was used as the coating material, and SPIONs coated with sericin have not been reported in the literature as a drug or gene carrier system.
The present study aimed to design and develop a novel siRNA carrier system based on SPIONs. We prepared and characterized a nanomaterial comprised of SPIONs covalently coated with a biocompatible protein, sericin (Ser), and modified with a cationic polymer, poly-l-lysine (PLL) to incorporate the negatively charged siRNA. Transmission electron microscopy (TEM) images revealed that obtained nanoparticles had narrow size distribution with spherical shape and coated nanoparticles presented black spheres in the SPION core surrounded with a gray layer, which indicated sericin coating. Adding PLL to Ser-SPIONs did not cause any dramatic change in nanoparticle size whereas it provided a net positive charge to the particles. Additionally, electron spin resonance (ESR) analysis showed that sericin coating and PLL modification did not efface the superparamagnetic properties of the particles. We successfully attached the control-siRNAs to the surface of PLL/Ser-SPIONs, forming siRNA-PLL/Ser-SPION nanoplatforms and achieving high siRNA binding efficiencies ranged between 81.90% and 93.50%. The ability of SPIONs-based nanocarriers to fulfill their promises in clinical use depends on keeping their nanotoxicity properties to a minimum. All the nanoparticle formulations tested herein were found to be biocompatible against non-cancerous and cancer cells by cytotoxicity assays. We also investigated the effect of resulting bare particles, PLL/Ser-SPIONs, on the clonogenicity of cancer cells. According to the results, the cells exhibited colony formation ability depending on the concentration of the nanoparticles and PLL/Ser-SPIONs did not block clonogenicity, except at very high doses. Taken together, our findings imply that these new PLL/Ser-SPIONs can be considered as a safe and promising carrier candidate to be used and developed for further siRNA-based applications.
中文翻译:

新型聚-L-赖氨酸修饰丝胶包被超顺磁性氧化铁纳米粒子作为siRNA载体的开发
小干扰 RNA (siRNA) 是一种很有前景的治疗方式,然而,由于缺乏安全有效的载体系统,其成功的临床应用仍然具有挑战性。基于超顺磁性氧化铁纳米粒子 (SPION) 的药物或基因载体系统在纳米医学中显示出巨大的前景。它们具有内在独特的超顺磁性,使它们能够集中在施加外部磁场的目标治疗作用部位。SPION 也可以设计为治疗诊断剂,以同时实现治疗和诊断目的。尽管有这些有利的特征,但 SPION 是胶体不稳定的,可以很容易地在循环中消除。更重要的是,与 SPION 相关的毒理学问题,这往往会导致活性氧 (ROS) 的产生,需要彻底考虑。迄今为止,已经提出了各种类型的聚合物来覆盖 SPION 的表面以克服这些缺点。蚕丝蛋白、丝胶由于其高生物相容性、良好的生物降解性和体内稳定性,是理想的包衣材料。在 SPIONs 作为 siRNA 载体的发展方面,据我们所知,没有使用蛋白质作为涂层材料,并且在文献中没有报道丝胶包裹的 SPIONs 作为药物或基因载体系统。和体内稳定性。在 SPIONs 作为 siRNA 载体的发展方面,据我们所知,没有使用蛋白质作为涂层材料,并且在文献中没有报道丝胶包裹的 SPIONs 作为药物或基因载体系统。和体内稳定性。在 SPIONs 作为 siRNA 载体的发展方面,据我们所知,没有使用蛋白质作为涂层材料,并且在文献中没有报道丝胶包裹的 SPIONs 作为药物或基因载体系统。
本研究旨在设计和开发一种基于 SPION 的新型 siRNA 载体系统。我们制备并表征了一种由 SPION 组成的纳米材料,该 SPION 用生物相容性蛋白质丝胶 (Ser) 共价包被,并用阳离子聚合物、poly- l修饰。-赖氨酸 (PLL) 以掺入带负电荷的 siRNA。透射电子显微镜 (TEM) 图像显示,获得的纳米颗粒具有窄的球形尺寸分布,并且包覆的纳米颗粒在 SPION 核心中呈现黑色球体,周围有灰色层,表明丝胶涂层。将 PLL 添加到 Ser-SPIONs 不会导致纳米颗粒尺寸发生任何显着变化,但它为颗粒提供了净正电荷。此外,电子自旋共振 (ESR) 分析表明,丝胶涂层和 PLL 修饰不会消除颗粒的超顺磁性。我们成功地将 control-siRNAs 连接到 PLL/Ser-SPIONs 的表面,形成了 siRNA-PLL/Ser-SPION 纳米平台,实现了 81.90% 到 93.50% 的高 siRNA 结合效率。基于 SPION 的纳米载体在临床应用中实现其承诺的能力取决于将其纳米毒性特性保持在最低限度。通过细胞毒性测定发现本文测试的所有纳米颗粒制剂对非癌性细胞和癌细胞具有生物相容性。我们还研究了所得裸粒子 PLL/Ser-SPIONs 对癌细胞克隆形成的影响。根据结果,细胞表现出集落形成能力取决于纳米颗粒的浓度,并且 PLL/Ser-SPIONs 不会阻止克隆形成,除非在非常高的剂量下。总之,我们的发现意味着这些新的 PLL/Ser-SPIONs 可以被认为是一种安全且有前途的载体候选物,可用于和开发用于进一步基于 siRNA 的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号