International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2021-09-24 , DOI: 10.1016/j.ijhydene.2021.09.044 Tongwen Huang 1 , Huang Liu 1 , Chengshang Zhou 1
|
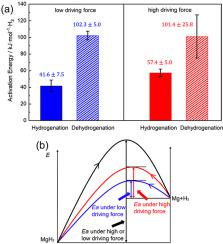
Catalyst doping is a critical approach to improve the kinetics of MgH2. However, reported activation energy () values of catalyzed MgH2 systems show a large discrepancy. This study investigates the effect of the driving force on for (de)hydrogenation reactions. The kinetic rate constant is calculated using Johnson-Mehl-Avrami-Kolomogorov (JMAK) model. is obtained according to a series of kinetic measurements under a nearly constant driving force. Under a low driving force , the for dehydrogenation and hydrogenation are calculated to be 102.35.0 and 41.67.5 kJ/mol H2, respectively. Under a high driving force , the for dehydrogenation and hydrogenation are calculated to be 101.425.8 and 57.45.0 kJ/mol H2, respectively. The result shows that the high driving force leads to higher hydrogenation over the obtained the low driving force, while dehydrogenation remains unchanged under different driving forces.
中文翻译:

驱动力对催化MgH2脱氢加氢活化能的影响
催化剂掺杂是改善 MgH 2动力学的关键方法。然而,报道的活化能() 催化 MgH 2系统的值显示出很大的差异。本研究探讨了驱动力对用于(脱)氢化反应。使用 Johnson-Mehl-Avrami-Kolomogorov (JMAK) 模型计算动力学速率常数。在几乎恒定的驱动力下根据一系列动力学测量获得。在低驱动力下, 这 脱氢加氢计算为102.35.0 和 41.6分别为 7.5 kJ/mol H 2。在高驱动力下, 这 脱氢加氢计算为101.425.8 和 57.4分别为5.0 kJ/mol H 2。结果表明,高驱动力导致更高的氢化 在 获得低驱动力,同时脱氢 在不同的驱动力下保持不变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号