Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-09-24 , DOI: 10.1016/j.jhep.2021.09.010 Vishal C Patel 1 , Sunjae Lee 2 , Mark J W McPhail 3 , Kevin Da Silva 4 , Susie Guilly 4 , Ane Zamalloa 5 , Elizabeth Witherden 6 , Sidsel Støy 7 , Godhev Kumar Manakkat Vijay 8 , Nicolas Pons 4 , Nathalie Galleron 4 , Xaiohong Huang 8 , Selin Gencer 9 , Muireann Coen 9 , Thomas Henry Tranah 10 , Julia Alexis Wendon 10 , Kenneth D Bruce 11 , Emmanuelle Le Chatelier 4 , Stanislav Dusko Ehrlich 4 , Lindsey Ann Edwards 8 , Saeed Shoaie 12 , Debbie Lindsay Shawcross 10
|
Background & Aims
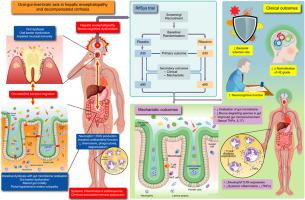
Rifaximin-α is efficacious for the prevention of recurrent hepatic encephalopathy (HE), but its mechanism of action remains unclear. We postulated that rifaximin-α reduces gut microbiota-derived endotoxemia and systemic inflammation, a known driver of HE.
Methods
In a placebo-controlled, double-blind, mechanistic study, 38 patients with cirrhosis and HE were randomised 1:1 to receive either rifaximin-α (550 mg BID) or placebo for 90 days. Primary outcome: 50% reduction in neutrophil oxidative burst (OB) at 30 days. Secondary outcomes: changes in psychometric hepatic encephalopathy score (PHES) and neurocognitive functioning, shotgun metagenomic sequencing of saliva and faeces, plasma and faecal metabolic profiling, whole blood bacterial DNA quantification, neutrophil toll-like receptor (TLR)-2/4/9 expression and plasma/faecal cytokine analysis.
Results
Patients were well-matched: median MELD (11 rifaximin-α vs. 10 placebo). Rifaximin-α did not lead to a 50% reduction in spontaneous neutrophil OB at 30 days compared to baseline (p = 0.48). However, HE grade normalised (p = 0.014) and PHES improved (p = 0.009) after 30 days on rifaximin-α. Rifaximin-α reduced circulating neutrophil TLR-4 expression on day 30 (p = 0.021) and plasma tumour necrosis factor-α (TNF-α) (p <0.001). Rifaximin-α suppressed oralisation of the gut, reducing levels of mucin-degrading sialidase-rich species, Streptococcus spp, Veillonella atypica and parvula, Akkermansia and Hungatella. Rifaximin-α promoted a TNF-α- and interleukin-17E-enriched intestinal microenvironment, augmenting antibacterial responses to invading pathobionts and promoting gut barrier repair. Those on rifaximin-α were less likely to develop infection (odds ratio 0.21; 95% CI 0.05-0.96).
Conclusion
Rifaximin-α led to resolution of overt and covert HE, reduced the likelihood of infection, reduced oralisation of the gut and attenuated systemic inflammation. Rifaximin-α plays a role in gut barrier repair, which could be the mechanism by which it ameliorates bacterial translocation and systemic endotoxemia in cirrhosis.
Clinical Trial Number
ClinicalTrials.gov NCT02019784.
Lay summary
In this clinical trial, we examined the underlying mechanism of action of an antibiotic called rifaximin-α which has been shown to be an effective treatment for a complication of chronic liver disease which effects the brain (termed encephalopathy). We show that rifaximin-α suppresses gut bacteria that translocate from the mouth to the intestine and cause the intestinal wall to become leaky by breaking down the protective mucus barrier. This suppression resolves encephalopathy and reduces inflammation in the blood, preventing the development of infection.
中文翻译:

利福昔明-α 可减少肝硬化和脑病中的肠源性炎症和粘蛋白降解:RIFSYS 随机对照试验
背景与目标
利福昔明-α可有效预防复发性肝性脑病(HE),但其作用机制尚不清楚。我们假设利福昔明-α 可减少肠道微生物群衍生的内毒素血症和全身炎症,这是 HE 的已知驱动因素。
方法
在一项安慰剂对照、双盲机制研究中,38 名患有肝硬化和 HE 的患者以 1:1 的比例随机接受利福昔明-α(550 mg BID)或安慰剂,为期 90 天。主要结果:30 天时中性粒细胞氧化爆发 (OB) 减少 50%。次要结果:心理测量性肝性脑病评分 (PHES) 和神经认知功能的变化、唾液和粪便的鸟枪法宏基因组测序、血浆和粪便代谢分析、全血细菌 DNA 定量、中性粒细胞 Toll 样受体 (TLR)-2/4/9表达和血浆/粪便细胞因子分析。
结果
患者匹配良好:中位 MELD(利福昔明-α 11 例vs安慰剂 10 例)。与基线相比,利福昔明-α 在 30 天时并未导致自发性中性粒细胞 OB 减少 50%( p = 0.48)。然而,使用利福昔明-α 30 天后,HE 等级正常化 ( p = 0.014),PHES 改善 ( p = 0.009)。利福昔明-α 在第 30 天降低循环中性粒细胞 TLR-4 表达 ( p = 0.021) 和血浆肿瘤坏死因子-α (TNF-α) ( p < 0.001)。利福昔明-α 抑制肠道的口服,降低富含粘蛋白降解唾液酸酶的物种、链球菌、非典型韦荣球菌和小球菌、阿克曼氏菌和亨加特氏菌的水平。利福昔明-α 促进富含 TNF-α 和白细胞介素 17E 的肠道微环境,增强对入侵病原体的抗菌反应并促进肠道屏障修复。服用利福昔明-α 的患者发生感染的可能性较小(比值比 0.21;95% CI 0.05-0.96)。
结论
利福昔明-α 可以解决显性和隐性 HE,降低感染的可能性,减少肠道的口腔吸收并减轻全身炎症。利福昔明-α 在肠道屏障修复中发挥作用,这可能是其改善肝硬化细菌移位和全身性内毒素血症的机制。
临床试验号
ClinicalTrials.gov NCT02019784。
外行总结
在这项临床试验中,我们研究了一种名为利福昔明-α 的抗生素的潜在作用机制,该抗生素已被证明可以有效治疗影响大脑的慢性肝病并发症(称为脑病)。我们发现,利福昔明-α 可抑制肠道细菌从口腔转移到肠道,并通过破坏保护性粘液屏障而导致肠壁渗漏。这种抑制可以解决脑病并减少血液中的炎症,从而防止感染的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号