Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New total synthesis and structure confirmation of putative (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ra06225e Lívia Dikošová 1 , Barbora Otočková 1 , Tomáš Malatinský 1 , Jana Doháňošová 2 , Mária Kopáčová 3 , Anna Ďurinová 4 , Lucie Smutná 4 , František Trejtnar 4 , Róbert Fischer 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ra06225e Lívia Dikošová 1 , Barbora Otočková 1 , Tomáš Malatinský 1 , Jana Doháňošová 2 , Mária Kopáčová 3 , Anna Ďurinová 4 , Lucie Smutná 4 , František Trejtnar 4 , Róbert Fischer 1
Affiliation
|
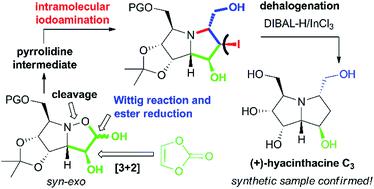
A unique synthesis of polyhydroxylated pyrrolizidine alkaloids, namely (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3 is presented. The strategy relies on a 1,3-dipolar cycloaddition of an L-mannose derived nitrone, which owing to its great syn-stereoselectivity builds up the majority of the required stereocenters. The following key steps include Wittig olefination and iodine-mediated aminocyclisation, that provide two epimeric pyrrolizidines with the appropriate configuration. As a result, structure and steric arrangement of the first synthetically prepared (+)-hyacinthacine C3 are proved to be correct, clearly confirming the inconsistency with the stereochemistry assigned to the natural sample. With respect to the previously proven glycosidase inhibitory activities, the antiproliferative effect of (+)-hyacinthacine C3 and (+)-5-epi-hyacinthacine C3 was evaluated using several cell line models.
中文翻译:

推定的 (+)-hyacinthacine C3 和 (+)-5-epi-hyacinthacine C3 的新全合成和结构确认
提出了一种独特的多羟基化吡咯里西啶生物碱合成方法,即 (+)-hyacinthacine C 3和 (+)-5- epi - hyacinthacine C 3。该策略依赖于L-甘露糖衍生的硝酮的 1,3-偶极环加成,由于其出色的顺式立体选择性,可形成大部分所需的立体中心。以下关键步骤包括 Wittig 烯化和碘介导的氨基环化,这提供了两种具有适当构型的差向异构吡咯里西啶。结果,第一个合成制备的(+)-hyacinthacine C 3的结构和空间排列被证明是正确的,清楚地证实了与分配给天然样品的立体化学的不一致。关于先前证实的糖苷酶抑制活性,(+)-hyacinthacine C 3和(+)-5- epi -hyacinthacine C 3的抗增殖作用使用几种细胞系模型进行了评估。
更新日期:2021-09-24
中文翻译:

推定的 (+)-hyacinthacine C3 和 (+)-5-epi-hyacinthacine C3 的新全合成和结构确认
提出了一种独特的多羟基化吡咯里西啶生物碱合成方法,即 (+)-hyacinthacine C 3和 (+)-5- epi - hyacinthacine C 3。该策略依赖于L-甘露糖衍生的硝酮的 1,3-偶极环加成,由于其出色的顺式立体选择性,可形成大部分所需的立体中心。以下关键步骤包括 Wittig 烯化和碘介导的氨基环化,这提供了两种具有适当构型的差向异构吡咯里西啶。结果,第一个合成制备的(+)-hyacinthacine C 3的结构和空间排列被证明是正确的,清楚地证实了与分配给天然样品的立体化学的不一致。关于先前证实的糖苷酶抑制活性,(+)-hyacinthacine C 3和(+)-5- epi -hyacinthacine C 3的抗增殖作用使用几种细胞系模型进行了评估。







































 京公网安备 11010802027423号
京公网安备 11010802027423号