Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electro-oxidative cyclization: access to quinazolinones via K2S2O8 without transition metal catalyst and base
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ra05092c Yongzhi Hu 1 , Huiqing Hou 1 , Ling Yu 2 , Sunying Zhou 1 , Xianghua Wu 3 , Weiming Sun 1 , Fang Ke 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-24 , DOI: 10.1039/d1ra05092c Yongzhi Hu 1 , Huiqing Hou 1 , Ling Yu 2 , Sunying Zhou 1 , Xianghua Wu 3 , Weiming Sun 1 , Fang Ke 1
Affiliation
|
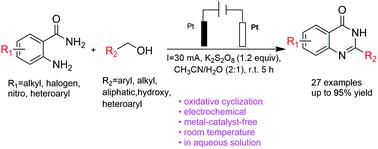
A K2S2O8-promoted oxidative tandem cyclization of primary alcohols with 2-aminobenzamides to synthesize quinazolinones was successfully achieved under undivided electrolytic conditions without a transition metal and base. The key feature of this protocol is the utilization of K2S2O8 as an inexpensive and easy-to-handle radical surrogate that can effectively promote the reaction via a simple procedure, leading to the formation of nitrogen heterocycles via direct oxidative cyclization at room temperature in a one-pot procedure under constant current. Owing to the use of continuous-flow electrochemical setups, this green, mild and practical electrosynthesis features high efficiency and excellent functional group tolerance and is easy to scale up.
中文翻译:

电氧化环化:在没有过渡金属催化剂和碱的情况下通过 K2S2O8 获得喹唑啉酮
AK 2 S 2 O 8促进了伯醇与2-氨基苯甲酰胺的氧化串联环化以合成喹唑啉酮,在没有过渡金属和碱的不分电解条件下成功实现。该协议的主要特点是利用 K 2 S 2 O 8作为一种廉价且易于处理的自由基替代物,可以通过简单的程序有效地促进反应,从而通过形成氮杂环在恒定电流下以一锅法在室温下直接氧化环化。由于使用了连续流动的电化学装置,这种绿色、温和、实用的电合成具有高效率和优异的官能团耐受性,并且易于放大。
更新日期:2021-09-24
中文翻译:

电氧化环化:在没有过渡金属催化剂和碱的情况下通过 K2S2O8 获得喹唑啉酮
AK 2 S 2 O 8促进了伯醇与2-氨基苯甲酰胺的氧化串联环化以合成喹唑啉酮,在没有过渡金属和碱的不分电解条件下成功实现。该协议的主要特点是利用 K 2 S 2 O 8作为一种廉价且易于处理的自由基替代物,可以通过简单的程序有效地促进反应,从而通过形成氮杂环在恒定电流下以一锅法在室温下直接氧化环化。由于使用了连续流动的电化学装置,这种绿色、温和、实用的电合成具有高效率和优异的官能团耐受性,并且易于放大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号