Tetrahedron ( IF 2.1 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.tet.2021.132476 Hao-Nan Wang 1 , Jing-Yan Dong 1 , Jin Shi 1 , Cheng-Pan Zhang 1
|
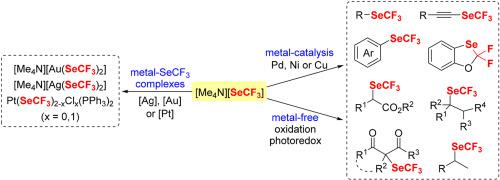
The trifluoromethylseleno group (SeCF3) possesses unique electrosteric effect and high lipophilicity and has been verified as a pharmaceutically relevant functionality in modulating the physicochemical and biological properties of drug-like molecules. The formation of C–SeCF3 bonds by reactions with [Me4N][SeCF3] under transition-metal-catalyzed or -free conditions has emerged in the last decade. [Me4N][SeCF3] proves to be a versatile and promising SeCF3 reagent among all trifluoromethylselenolation reagents in the synthesis of CF3Se-containing compounds, which is thermally stable, readily accessible, non-volatile and easy to control. This review describes the advances of trifluoromethylselenolation reactions with [Me4N][SeCF3] and is divided into three parts (synthesis of metal-SeCF3 complexes with [Me4N][SeCF3], transition-metal-catalyzed trifluoromethylselenolations with [Me4N][SeCF3], and transition-metal-free trifluoromethylselenolations with [Me4N][SeCF3]). Although the use of [Me4N][SeCF3] has achieved a variety of trifluoromethylselenolation reactions, the approaches for C–SeCF3 bond formation are still lacking in comparison with the homologous C-OCF3 and C-SCF3 bonds formation. We anticipate that continuous efforts will focus on the development of more widely applicable trifluoromethylselenolation reactions with [Me4N][SeCF3] by transition-metal catalysis, transition-metal-free conditions, oxidation, or photoredox strategies. Since there have been no CF3Se-containing pharmaceuticals used in clinical trials so far, efforts will also be devoted to exploring the biological activities of trifluoromethylselenolated compounds in the future for discovery of CF3Se-drugs with big challenges as well as great opportunities.
中文翻译:

使用多功能 [Me4N][SeCF3] 试剂的三氟甲基硒化反应
三氟甲基硒基团 (SeCF 3 ) 具有独特的电空间效应和高亲脂性,已被证实在调节类药物分子的理化和生物学特性方面具有药学相关功能。在过去十年中出现了通过在过渡金属催化或无条件下与 [Me 4 N][SeCF 3 ]反应形成 C-SeCF 3键。[Me 4 N][SeCF 3 ]被证明是CF 3合成中所有三氟甲基硒化试剂中的通用且有前途的SeCF 3试剂含硒化合物,具有热稳定性,易于获取,不挥发且易于控制。本综述描述了[Me 4 N][SeCF 3 ]三氟甲基硒化反应的进展,分为三个部分(金属-SeCF 3与[Me 4 N][SeCF 3 ]配合物的合成,过渡金属催化的三氟甲基硒化与[Me 4 N][SeCF 3 ] [Me 4 N][SeCF 3 ],以及无过渡金属的三氟甲基硒化与 [Me 4 N][SeCF 3 ])。虽然使用 [Me 4 N][SeCF 3] 已经实现了多种三氟甲基硒化反应,与同源的 C-OCF 3和 C-SCF 3键的形成相比,C-SeCF 3键的形成方法仍然缺乏。我们预计,通过过渡金属催化、无过渡金属条件、氧化或光氧化还原策略,将继续努力开发更广泛适用的与 [Me 4 N][SeCF 3 ] 的三氟甲基硒化反应。由于目前尚无含CF 3 Se 的药物用于临床试验,未来还将致力于探索三氟甲基硒化化合物的生物活性以发现CF3 Se-drugs 大挑战也大机遇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号