Tetrahedron ( IF 2.1 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.tet.2021.132457 Fritz Schömberg 1 , Milica Perić 1 , Maximilian Meyer 1 , Ivan Vilotijević 1
|
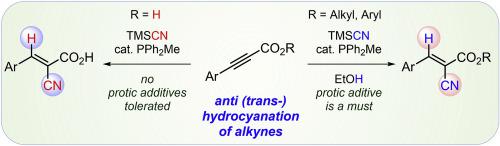
trans-Selective hydrocyanation of ynoates and ynones in the presence of TMSCN and an alcohol additive are catalyzed by nucleophilic phosphines. The trisubstituted E-olefin products of anti-addition of hydrogen cyanide to the alkyne are produced with high regio- and stereoselectivity. The alcohol additive reacts with TMSCN to produce hydrogen cyanide in situ. Ynoic acids undergo the phosphine catalyzed hydrocyanation in the presence of TMSCN under aprotic conditions only. In these reactions, TMSCN reacts with the acid to generate hydrogen cyanide and the silyl ester which, unlike the acid, undergoes phosphine catalyzed hydrocyanation and gives the stereo-defined E-2-cyano-acrylic acids after work up.
中文翻译:

亲核膦催化的炔诺酸酯、炔诺酮和炔诺酸的反式选择性氢氰化
在 TMSCN 和醇添加剂存在下,ynoates 和ynones 的反式选择性氢氰化反应由亲核膦催化。氰化氢反加成到炔烃的三取代E-烯烃产物具有高区域选择性和立体选择性。醇类添加剂与 TMSCN 反应,原位生成氰化氢。仅在非质子惰性条件下,在 TMSCN 存在下,炔酸经历膦催化的氢氰化。在这些反应中,TMSCN 与酸反应生成氰化氢和甲硅烷基酯,与酸不同,后者经过膦催化氢氰化并在后处理后生成立体定义的E -2-氰基丙烯酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号