Structure ( IF 4.4 ) Pub Date : 2021-09-24 , DOI: 10.1016/j.str.2021.09.005 Johannes Schimpf 1 , Sabrina Oppermann 1 , Tatjana Gerasimova 2 , Ana Filipa Santos Seica 3 , Petra Hellwig 4 , Irina Grishkovskaya 5 , Daniel Wohlwend 1 , David Haselbach 5 , Thorsten Friedrich 1
|
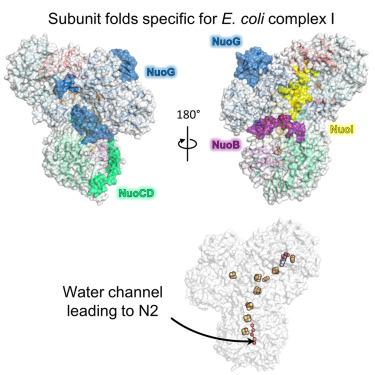
Respiratory complex I drives proton translocation across energy-transducing membranes by NADH oxidation coupled with (ubi)quinone reduction. In humans, its dysfunction is associated with neurodegenerative diseases. The Escherichia coli complex represents the structural minimal form of an energy-converting NADH:ubiquinone oxidoreductase. Here, we report the structure of the peripheral arm of the E. coli complex I consisting of six subunits, the FMN cofactor, and nine iron-sulfur clusters at 2.7 Å resolution obtained by cryo electron microscopy. While the cofactors are in equivalent positions as in the complex from other species, individual subunits are adapted to the absence of supernumerary proteins to guarantee structural stability. The catalytically important subunits NuoC and D are fused resulting in a specific architecture of functional importance. Striking features of the E. coli complex are scrutinized by mutagenesis and biochemical characterization of the variants. Moreover, the arrangement of the subunits sheds light on the unknown assembly of the complex.
中文翻译:

简约呼吸复合体 I 外周臂的结构
呼吸复合物 I 通过 NADH 氧化和(泛)醌还原驱动质子在能量转导膜上的易位。在人类中,其功能障碍与神经退行性疾病有关。大肠杆菌复合物代表能量转换 NADH:泛醌氧化还原酶的结构最小形式。在这里,我们报告了大肠杆菌外周臂的结构复合物 I 由六个亚基、FMN 辅因子和九个铁硫簇组成,分辨率为 2.7 Å,通过低温电子显微镜获得。虽然辅因子与其他物种的复合物中的位置相同,但单个亚基适应不存在多余蛋白质以保证结构稳定性。催化重要的亚基 NuoC 和 D 融合在一起,形成具有功能重要性的特定结构。大肠杆菌复合物的显着特征通过变体的诱变和生化表征进行了仔细检查。此外,亚基的排列揭示了复合体的未知组装。











































 京公网安备 11010802027423号
京公网安备 11010802027423号