当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Cyclic 1,3-Diene Forming Acyl-Coenzyme A Dehydrogenases
ChemBioChem ( IF 2.6 ) Pub Date : 2021-09-23 , DOI: 10.1002/cbic.202100421 Johannes W Kung 1 , Anne-Katrin Meier 1 , Max Willistein 1 , Sina Weidenweber 2 , Ulrike Demmer 2 , Ulrich Ermler 2 , Matthias Boll 1
ChemBioChem ( IF 2.6 ) Pub Date : 2021-09-23 , DOI: 10.1002/cbic.202100421 Johannes W Kung 1 , Anne-Katrin Meier 1 , Max Willistein 1 , Sina Weidenweber 2 , Ulrike Demmer 2 , Ulrich Ermler 2 , Matthias Boll 1
Affiliation
|
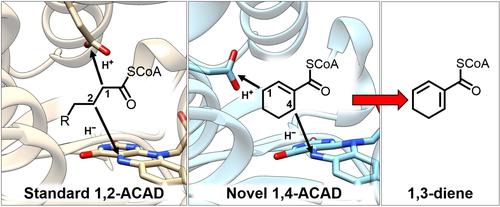
Expanding the biocatalytic repertoire: Canonical acyl-coenzyme dehydrogenases catalyze the 1,2-dehydrogenation of aliphatic thioesters in a highly conserved manner with a invariant glutamate serving as catalytic base and a flavin as hydride acceptor. By removal of this glutamate and positioning of a novel aspartate base at C3, an 1,4 dehydrogenation activity is achieved, thereby generating a cyclic 1,3-diene building block.

中文翻译:

环状 1,3-二烯形成酰基辅酶 A 脱氢酶的结构基础
扩展生物催化库:典型的酰基辅酶脱氢酶以高度保守的方式催化脂肪族硫酯的 1,2-脱氢,其中不变的谷氨酸盐作为催化碱,黄素作为氢化物受体。通过去除这种谷氨酸并将新的天冬氨酸碱基定位在 C3,实现了 1,4 脱氢活性,从而产生了环状 1,3-二烯结构单元。

更新日期:2021-11-16

中文翻译:

环状 1,3-二烯形成酰基辅酶 A 脱氢酶的结构基础
扩展生物催化库:典型的酰基辅酶脱氢酶以高度保守的方式催化脂肪族硫酯的 1,2-脱氢,其中不变的谷氨酸盐作为催化碱,黄素作为氢化物受体。通过去除这种谷氨酸并将新的天冬氨酸碱基定位在 C3,实现了 1,4 脱氢活性,从而产生了环状 1,3-二烯结构单元。












































 京公网安备 11010802027423号
京公网安备 11010802027423号