当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the scope of native chemical ligation – templated small molecule drug synthesis via benzanilide formation
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc00513h Richard Houska 1 , Marvin Björn Stutz 1 , Oliver Seitz 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc00513h Richard Houska 1 , Marvin Björn Stutz 1 , Oliver Seitz 1
Affiliation
|
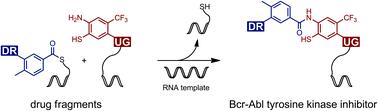
We describe a reaction system that enables the synthesis of Bcr–Abl tyrosine kinase inhibitors (TKI) via benzanilide formation in water. The reaction is based on native chemical ligation (NCL). In contrast to previous applications, we used the NCL chemistry to establish aromatic rather than aliphatic amide bonds in coupling reactions between benzoyl and o-mercaptoaniline fragments. The method was applied for the synthesis of thiolated ponatinib and GZD824 derivatives. Acid treatment provided benzothiazole structures, which opens opportunities for diversification. Thiolation affected the affinity for Abl1 kinase only moderately. Of note, a ponatinib-derived benzothiazole also showed nanomolar affinity. NCL-enabled benzanilide formation may prove useful for fragment-based drug discovery. To show that benzanilide synthesis can be put under the control of a template, we connected the benzoyl and o-mercaptoaniline fragments to DNA and peptide nucleic acid (PNA) oligomers. Complementary RNA templates enabled adjacent binding of reactive conjugates triggering a rapid benzoyl transfer from a thioester-linked DNA conjugate to an o-mercaptoaniline-DNA or -PNA conjugate. We evaluated the influence of linker length and unpaired spacer nucleotides within the RNA template on the product yield. The data suggest that nucleic acid-templated benzanilide formation could find application in the establishment of DNA-encoded combinatorial libraries (DEL).
中文翻译:

扩大天然化学连接的范围——通过苯甲酰苯胺形成的模板化小分子药物合成
我们描述了一种反应系统,该系统能够通过在水中形成苯甲酰苯胺来合成 Bcr-Abl 酪氨酸激酶抑制剂 (TKI) 。该反应基于天然化学连接 (NCL)。与之前的应用相比,我们使用 NCL 化学在苯甲酰基和邻苯二甲酸之间的偶联反应中建立芳香族而非脂肪族酰胺键-巯基苯胺片段。该方法用于合成巯基普纳替尼和GZD824衍生物。酸处理提供了苯并噻唑结构,这为多样化提供了机会。硫醇化仅适度影响 Abl1 激酶的亲和力。值得注意的是,普纳替尼衍生的苯并噻唑也表现出纳摩尔亲和力。NCL 支持的苯甲酰苯胺的形成可能对基于片段的药物发现有用。为了表明苯甲酰苯胺的合成可以在模板的控制下进行,我们将苯甲酰基和o-巯基苯胺片段连接到 DNA 和肽核酸 (PNA) 寡聚体。互补的 RNA 模板能够使反应性偶联物的相邻结合触发苯甲酰从硫酯连接的 DNA 偶联物快速转移到o-巯基苯胺-DNA或-PNA偶联物。我们评估了接头长度和 RNA 模板内未配对的间隔核苷酸对产品产量的影响。数据表明,核酸模板化苯甲酰苯胺的形成可以应用于建立 DNA 编码的组合文库 (DEL)。
更新日期:2021-09-23
中文翻译:

扩大天然化学连接的范围——通过苯甲酰苯胺形成的模板化小分子药物合成
我们描述了一种反应系统,该系统能够通过在水中形成苯甲酰苯胺来合成 Bcr-Abl 酪氨酸激酶抑制剂 (TKI) 。该反应基于天然化学连接 (NCL)。与之前的应用相比,我们使用 NCL 化学在苯甲酰基和邻苯二甲酸之间的偶联反应中建立芳香族而非脂肪族酰胺键-巯基苯胺片段。该方法用于合成巯基普纳替尼和GZD824衍生物。酸处理提供了苯并噻唑结构,这为多样化提供了机会。硫醇化仅适度影响 Abl1 激酶的亲和力。值得注意的是,普纳替尼衍生的苯并噻唑也表现出纳摩尔亲和力。NCL 支持的苯甲酰苯胺的形成可能对基于片段的药物发现有用。为了表明苯甲酰苯胺的合成可以在模板的控制下进行,我们将苯甲酰基和o-巯基苯胺片段连接到 DNA 和肽核酸 (PNA) 寡聚体。互补的 RNA 模板能够使反应性偶联物的相邻结合触发苯甲酰从硫酯连接的 DNA 偶联物快速转移到o-巯基苯胺-DNA或-PNA偶联物。我们评估了接头长度和 RNA 模板内未配对的间隔核苷酸对产品产量的影响。数据表明,核酸模板化苯甲酰苯胺的形成可以应用于建立 DNA 编码的组合文库 (DEL)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号