当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stimuli-Responsive Polycycles Based on Hetero-Buckybowl Trithiasumanene
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-09-22 , DOI: 10.1002/cjoc.202100578 Wenbo Wang 1 , Lijun Feng 1 , Xinqiang Hua 1 , Chengshan Yuan 1 , Xiangfeng Shao 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-09-22 , DOI: 10.1002/cjoc.202100578 Wenbo Wang 1 , Lijun Feng 1 , Xinqiang Hua 1 , Chengshan Yuan 1 , Xiangfeng Shao 1
Affiliation
|
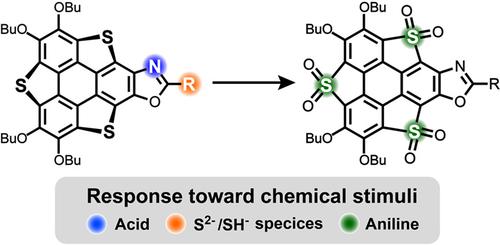
Stimuli-responsive molecules are highly desirable in different scenario such as smart materials, bio-imaging, and environment monitoring. Herein, a series of hetero polycycles which show optical response toward chemical stimuli are synthesized from trithiasumanene (TTS). The TTS is transformed into ortho-quinone form, which then undergoes three-component Debus-Radziszewski reaction with aldehydes and ammonia to give oxazole-fused TTS (2—6). The thiophene rings on 3—5 are selectively oxidized to thiophene-S,S-dioxides, affording 3-3O2—5-3O2. It is found that the electronic structures of these compounds are governed by the substituents on oxazole moiety and oxidation state of thiophene ring. Moreover, these hetero polycycles exhibit optical response toward different chemical stimuli. Particularly, compounds 6 and 3-3O2 can serve as fluorescence detectors for harmful chemicals sulfide ions (S2–/HS–) and aniline, respectively. This work indicates that TTS is a promising precursor for the creation of responsive materials.
中文翻译:

基于杂巴基碗三硫马烯的刺激响应多环
刺激响应分子在智能材料、生物成像和环境监测等不同场景中非常受欢迎。在这里,一系列对化学刺激表现出光学响应的杂多环是由三硫杂环胺 (TTS) 合成的。TTS 转化为邻醌形式,然后与醛和氨发生三组分 Debus-Radziszewski 反应,得到恶唑稠合的 TTS (2-6)。3-5 上的噻吩环被选择性氧化为噻吩-S,S-二氧化物,得到 3-3O 2 —5-3O 2. 发现这些化合物的电子结构受恶唑部分的取代基和噻吩环的氧化态控制。此外,这些杂多环对不同的化学刺激表现出光学响应。特别是化合物 6 和 3-3O 2可分别用作有害化学物质硫化物离子 (S 2– /HS – ) 和苯胺的荧光检测器。这项工作表明 TTS 是创建响应材料的有前途的先驱。
更新日期:2021-11-15

中文翻译:

基于杂巴基碗三硫马烯的刺激响应多环
刺激响应分子在智能材料、生物成像和环境监测等不同场景中非常受欢迎。在这里,一系列对化学刺激表现出光学响应的杂多环是由三硫杂环胺 (TTS) 合成的。TTS 转化为邻醌形式,然后与醛和氨发生三组分 Debus-Radziszewski 反应,得到恶唑稠合的 TTS (2-6)。3-5 上的噻吩环被选择性氧化为噻吩-S,S-二氧化物,得到 3-3O 2 —5-3O 2. 发现这些化合物的电子结构受恶唑部分的取代基和噻吩环的氧化态控制。此外,这些杂多环对不同的化学刺激表现出光学响应。特别是化合物 6 和 3-3O 2可分别用作有害化学物质硫化物离子 (S 2– /HS – ) 和苯胺的荧光检测器。这项工作表明 TTS 是创建响应材料的有前途的先驱。












































 京公网安备 11010802027423号
京公网安备 11010802027423号