Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.molliq.2021.117624 Mehdi Shakourian-Fard 1 , Hamid Reza Ghenaatian 2 , Vali Alizadeh 3 , Ganesh Kamath 4 , Behzad Khalili 5
|
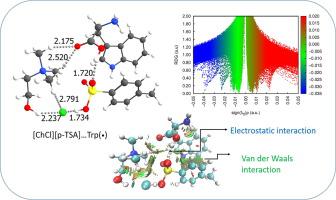
Processing of amino acids (AAs) is a very cost-intensive process. Recently, Li et al. used supported liquid membranes (SLMs) based on deep eutectic solvents (DESs) for amino-acid extraction and found that [choline chloride][p-Toluenesulfonic acid] ([ChCl][p-TSA])) DES was efficiently separating Tryptophan (Trp(•)) amino acid. Inspired by the study, here we investigate the interaction of [ChCl][p-TSA] DES with three forms of amino acids, including neutral(•), cationic(+), and anionic(-) forms using density functional theory (DFT) method at the M06-2X/6–311++G(d,p) level of theory. Our results indicate that the interaction of AAs(•/+/-) with [ChCl][p-TSA] DES is governed by the hydrogen bonding interactions, which is consistent with the findings of 1H NMR spectra by Li et al. Thermochemistry calculations indicate that the formation of [ChCl][p-TSA]…AAs(•/+/-) complexes is an exothermic and favorable reaction and proceeds spontaneously. Among the three forms of AAs(•/+/-), cationic forms (AAs(+)) exhibited the most tendency to interact with the [ChCl][p-TSA] DES. The binding energy (ΔEb) calculations show that Trp(•) has the strongest interaction with [ChCl][p-TSA] DES among the AAs(•), in agreement with the experimental results reported in the literature. The Gly(+) and Val(-) also have the highest ΔEb value with [ChCl][p-TSA] DES. A comparison between the calculated free energies and the extraction efficiency (%) reported by Li et al. for extraction of AAs(•) by [ChCl][p-TSA] DES indicates that the higher the free energy value, the higher is the extraction efficiency (%) of neutral AAs(•). Furthermore, the natural bond orbital (NBO) analysis, atoms in molecules (AIM) theory, and noncovalent interaction (NCI) plots were performed to determine the nature and strength of hydrogen bonding interactions between the [ChCl][p-TSA] DES and AAs(•/+/-). These analyses indicate that the O-H..O, O-H…[Cl]−, and N-H…[Cl]− interactions in the complexes are stronger than the N-H…O and C-H…O interactions. Energy decomposition analysis (EDA) method revealed that the charged AAs(+/-) have a greater tendency to interact with [ChCl][p-TSA] DES due to the presence of positive and negative charges on the AAs(+/-), which leads to increased strength in the electrostatic interactions between the AAs(+/-) and [ChCl][p-TSA] DES.
中文翻译:

深共熔溶剂与氨基酸相互作用的密度泛函理论研究
氨基酸 (AA) 的加工是一个成本非常高的过程。最近,李等人。使用基于深共熔溶剂 (DESs) 的支持液膜 (SLM) 进行氨基酸提取,发现 [氯化胆碱][对甲苯磺酸] ([ChCl][p-TSA])) DES 可有效分离色氨酸( Trp(•)) 氨基酸。受该研究的启发,我们在这里使用密度泛函理论 (DFT) 研究了 [ChCl][p-TSA] DES 与三种形式的氨基酸的相互作用,包括中性 (•)、阳离子 (+) 和阴离子 (-) 形式) 方法在 M06-2X/6–311++G(d,p) 理论水平。我们的结果表明 AA(•/+/-) 与 [ChCl][p-TSA] DES 的相互作用受氢键相互作用的控制,这与1Li 等人的 H NMR 光谱。热化学计算表明,[ChCl][p-TSA]…AAs(•/+/-) 配合物的形成是一种放热且有利的反应,并且会自发进行。在三种形式的 AA(•/+/-) 中,阳离子形式 (AAs(+)) 表现出与 [ChCl][p-TSA] DES 相互作用的最大趋势。结合能 (ΔE b ) 计算表明,Trp(•) 在 AA(•) 中与 [ChCl][p-TSA] DES 的相互作用最强,这与文献报道的实验结果一致。Gly(+) 和 Val(-) 也有最高的 ΔE b[ChCl][p-TSA] DES 的值。Li 等人 报告的计算自由能和提取效率 (%) 之间的比较。[ChCl][p-TSA] DES 提取 AA(•) 表明自由能值越高,中性 AA(•) 的提取效率 (%) 越高。此外,还进行了自然键轨道 (NBO) 分析、分子中原子 (AIM) 理论和非共价相互作用 (NCI) 图,以确定 [ChCl][p-TSA] DES 和AA(•/+/-)。这些分析表明 OH..O、OH…[Cl] -和 NH…[Cl] -配合物中的相互作用强于 NH…O 和 CH…O 相互作用。能量分解分析 (EDA) 方法表明,由于 AA(+/-) 上存在正电荷和负电荷,带电的 AA(+/-) 更倾向于与 [ChCl][p-TSA] DES 相互作用,这导致 AA(+/-) 和 [ChCl][p-TSA] DES 之间静电相互作用的强度增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号