Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.jconrel.2021.09.021 Mao-Ze Wang 1 , Ting-Wei Gu 1 , Yang Xu 1 , Lu Yang 1 , Zhi-Hong Jiang 2 , Li-Hua Peng 3
|
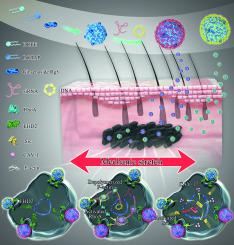
Gene therapy has gained popularity in the treatment of incurable diseases. However, cell components, such as surface membrane, cytoskeleton protein, and nuclear envelope, retard the transport of nucleic acids, lowering the transfection efficiency. We developed a physical-chemical hybrid platform (S-RCLs) involving cationic lipid nanoparticles (RCLs) exposed to cyclic stretch. The transfection efficiency and delivery mechanisms of S-RCLs for siRNAs and pDNAs (plasmid DNAs encoding luciferase) were investigated. S-RCLs effectively delivered both siRNAs and pDNAs by overcoming the cell barriers. Mechanistically, S-RCLs promote the cellular uptake mediated by CD44, EH-domain containing 2 (EHD2), and caveolin-1 (CAV-1); intracellular transport via MAP6 Domain Containing 1 (Map6d1) and F-actin; and DNA transcription regulated by LSM3 and Hist1h3e in the nucleus. Thus, S-RCLs are a promising hybrid platform with excellent efficiency and biocompatibility for gene delivery both in vitro and in vivo.
中文翻译:

用于核酸递送的细胞和脂质纳米颗粒的机械拉伸
基因疗法在不治之症的治疗中越来越受欢迎。然而,细胞成分,如表面膜、细胞骨架蛋白和核膜,会阻碍核酸的转运,降低转染效率。我们开发了一种物理-化学混合平台 (S-RCLs),涉及暴露于循环拉伸的阳离子脂质纳米粒子 (RCLs)。研究了 S-RCL 对 siRNA 和 pDNA(编码荧光素酶的质粒 DNA)的转染效率和传递机制。S-RCLs 通过克服细胞屏障有效地传递 siRNAs 和 pDNAs。从机制上讲,S-RCL 促进由 CD44、含 EH 结构域 2 (EHD2) 和 caveolin-1 (CAV-1) 介导的细胞摄取;细胞内运输通过包含 1 (Map6d1) 和 F-肌动蛋白的 MAP6 结构域;和 DNA 转录由细胞核中的 LSM3 和 Hist1h3e 调节。因此,S-RCLs 是一种很有前途的混合平台,具有出色的效率和生物相容性,可用于体外和体内基因传递。











































 京公网安备 11010802027423号
京公网安备 11010802027423号