当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights on Alanine and Arginine Binding to Silica with Atomic Resolution
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.jpclett.1c02398 Stefan Rauwolf 1 , Saientan Bag 2 , Rodrigo Rouqueiro 3 , Sebastian Patrick Schwaminger 1 , Ana Cristina Dias-Cabral 3 , Sonja Berensmeier 1 , Wolfgang Wenzel 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.jpclett.1c02398 Stefan Rauwolf 1 , Saientan Bag 2 , Rodrigo Rouqueiro 3 , Sebastian Patrick Schwaminger 1 , Ana Cristina Dias-Cabral 3 , Sonja Berensmeier 1 , Wolfgang Wenzel 2
Affiliation
|
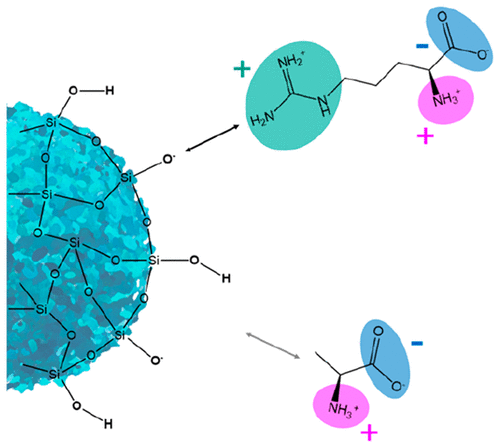
Interactions of biomolecules with inorganic oxide surfaces such as silica in aqueous solutions are of profound interest in various research fields, including chemistry, biotechnology, and medicine. While there is a general understanding of the dominating electrostatic interactions, the binding mechanism is still not fully understood. Here, chromatographic zonal elution and flow microcalorimetry experiments were combined with molecular dynamic simulations to describe the interaction of different capped amino acids with the silica surface. We demonstrate that ion pairing is the dominant electrostatic interaction. Surprisingly, the interaction strength is more dependent on the repulsive carboxy group than on the attracting amino group. These findings are essential for conducting experimental and simulative studies on amino acids when transferring the results to biomolecule–surface interactions.
中文翻译:

对丙氨酸和精氨酸以原子分辨率与二氧化硅结合的见解
生物分子与无机氧化物表面(如水溶液中的二氧化硅)的相互作用在包括化学、生物技术和医学在内的各个研究领域都具有深远的意义。虽然对主要的静电相互作用有了一般的了解,但结合机制仍未完全了解。在这里,色谱分区洗脱和流动微量热法实验与分子动力学模拟相结合,以描述不同封端氨基酸与硅胶表面的相互作用。我们证明离子对是主要的静电相互作用。令人惊讶的是,相互作用强度更依赖于排斥性羧基而不是吸引性氨基。
更新日期:2021-09-30
中文翻译:

对丙氨酸和精氨酸以原子分辨率与二氧化硅结合的见解
生物分子与无机氧化物表面(如水溶液中的二氧化硅)的相互作用在包括化学、生物技术和医学在内的各个研究领域都具有深远的意义。虽然对主要的静电相互作用有了一般的了解,但结合机制仍未完全了解。在这里,色谱分区洗脱和流动微量热法实验与分子动力学模拟相结合,以描述不同封端氨基酸与硅胶表面的相互作用。我们证明离子对是主要的静电相互作用。令人惊讶的是,相互作用强度更依赖于排斥性羧基而不是吸引性氨基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号