Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Central Composite Design for Adsorption of Pb(II) and Zn(II) Metals on PKM-2 Moringa oleifera Leaves
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c03069 Neethu Jayan 1 , Laxmi Deepak Bhatlu M 1 , Saufishan Thalikassery Akbar 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c03069 Neethu Jayan 1 , Laxmi Deepak Bhatlu M 1 , Saufishan Thalikassery Akbar 1
Affiliation
|
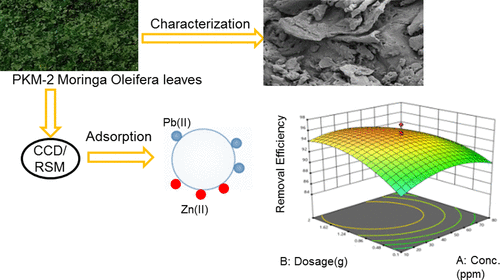
Biosorption is a very effective technique to eliminate the heavy metals present in the wastewater that utilize nongrowing biomass. The adsorption ability of the Periyakulam-2 (PKM-2) variety of Moringa Oleifera leaves (MOLs) to eliminate Pb(II) and Zn(II) ions from an aqueous solution was examined in this work. Fourier transform infrared (FTIR) spectroscopy, field-emission scanning electron microscopy, energy-dispersive X-ray (EDX) analysis, X-ray powder diffraction, and Brunauer–Emmett–Teller methods were used to characterize the PKM-2 variety of MOLs. The set of variables consists of the metal ion initial concentration, a dosage of the adsorbent, and pH were optimized with the help of the response surface methodology to get maximum metal removal efficiency of lead and zinc metals using the PKM-2 MOL biosorbent. A maximum Pb(II) removal of 95.6% was obtained under the condition of initial concentration of metal ions 38 mg/L, a dosage of the adsorbent 1.5 g, and pH 4.7, and a maximum zinc removal of 89.35% was obtained under the condition of initial concentration of metal ions 70 mg/L, a dosage of the adsorbent 0.6 g, and pH 3.2. The presence of lead and zinc ions on the biosorbent surface and the functional groups involved in the adsorption process were revealed using EDX and FTIR analysis, respectively. The adsorption data were evaluated by employing different isotherm and kinetic models. Among the isotherm models, Langmuir’s isotherm showed that the best fit and maximum adsorption capacities are 51.71 and 38.50 mg/g for lead and zinc, respectively. Kinetic studies showed accordance with the pseudo-second-order model to lead and zinc metal adsorption. Thermodynamic parameters confirmed (ΔG° < 0, ΔH° < 0, and ΔS° > 0) that the sorption mechanism is physisorption, exothermic, spontaneous, and favorable for adsorption. The results from this study show that the MOL of the PKM-2 type is a promising alternative for an ecofriendly, low-cost biosorbent that can effectively remove lead and zinc metals from aqueous solutions.
中文翻译:

PKM-2辣木叶片吸附Pb(II)和Zn(II)金属的中心复合设计
生物吸附是消除废水中存在的重金属的一种非常有效的技术,这些重金属利用非生长的生物质。辣木Periyakulam-2 (PKM-2) 品种的吸附能力在这项工作中检查了从水溶液中去除 Pb(II) 和 Zn(II) 离子的叶 (MOL)。傅里叶变换红外 (FTIR) 光谱、场发射扫描电子显微镜、能量色散 X 射线 (EDX) 分析、X 射线粉末衍射和 Brunauer-Emmett-Teller 方法用于表征 PKM-2 各种 MOL . 变量集包括金属离子初始浓度、吸附剂剂量和 pH,在响应面方法的帮助下进行了优化,以使用 PKM-2 MOL 生物吸附剂获得最大的铅和锌金属去除效率。在金属离子初始浓度38 mg/L、吸附剂用量1.5 g、pH 4.7的条件下,Pb(II)去除率最高为95.6%,锌去除率最高为89。在金属离子初始浓度70 mg/L、吸附剂用量0.6 g、pH 3.2的条件下得到35%。分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ 分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ 分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ Langmuir 等温线表明,铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ Langmuir 等温线表明,铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(ΔG °<0,ΔH °<0,ΔS °>0)表明吸附机理为物理吸附、放热、自发、有利于吸附。这项研究的结果表明,PKM-2 型 MOL 是一种有前途的环保、低成本生物吸附剂替代品,可以有效地从水溶液中去除铅和锌金属。
更新日期:2021-10-06
中文翻译:

PKM-2辣木叶片吸附Pb(II)和Zn(II)金属的中心复合设计
生物吸附是消除废水中存在的重金属的一种非常有效的技术,这些重金属利用非生长的生物质。辣木Periyakulam-2 (PKM-2) 品种的吸附能力在这项工作中检查了从水溶液中去除 Pb(II) 和 Zn(II) 离子的叶 (MOL)。傅里叶变换红外 (FTIR) 光谱、场发射扫描电子显微镜、能量色散 X 射线 (EDX) 分析、X 射线粉末衍射和 Brunauer-Emmett-Teller 方法用于表征 PKM-2 各种 MOL . 变量集包括金属离子初始浓度、吸附剂剂量和 pH,在响应面方法的帮助下进行了优化,以使用 PKM-2 MOL 生物吸附剂获得最大的铅和锌金属去除效率。在金属离子初始浓度38 mg/L、吸附剂用量1.5 g、pH 4.7的条件下,Pb(II)去除率最高为95.6%,锌去除率最高为89。在金属离子初始浓度70 mg/L、吸附剂用量0.6 g、pH 3.2的条件下得到35%。分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ 分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ 分别使用 EDX 和 FTIR 分析揭示了生物吸附剂表面上铅和锌离子的存在以及参与吸附过程的官能团。通过采用不同的等温线和动力学模型评估吸附数据。在等温线模型中,Langmuir 等温线显示铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ Langmuir 等温线表明,铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(Δ Langmuir 等温线表明,铅和锌的最佳拟合和最大吸附容量分别为 51.71 和 38.50 mg/g。动力学研究表明铅锌金属吸附符合准二级模型。热力学参数确认(ΔG °<0,ΔH °<0,ΔS °>0)表明吸附机理为物理吸附、放热、自发、有利于吸附。这项研究的结果表明,PKM-2 型 MOL 是一种有前途的环保、低成本生物吸附剂替代品,可以有效地从水溶液中去除铅和锌金属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号