Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoenzymatic and Protecting-Group-Free Synthesis of 1,4-Substituted 1,2,3-Triazole-α-d-glucosides with Potent Inhibitory Activity toward Lysosomal α-Glucosidase
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-22 , DOI: 10.1021/acsomega.1c03928 Jaggaiah N Gorantla 1 , Santhi Maniganda 1 , Salila Pengthaisong 1 , Lukana Ngiwsara 2 , Phannee Sawangareetrakul 2 , Suwadee Chokchaisiri 1 , Prasat Kittakoop 3 , Jisnuson Svasti 2 , James R Ketudat Cairns 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-22 , DOI: 10.1021/acsomega.1c03928 Jaggaiah N Gorantla 1 , Santhi Maniganda 1 , Salila Pengthaisong 1 , Lukana Ngiwsara 2 , Phannee Sawangareetrakul 2 , Suwadee Chokchaisiri 1 , Prasat Kittakoop 3 , Jisnuson Svasti 2 , James R Ketudat Cairns 1, 2
Affiliation
|
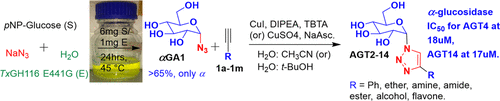
α-Glucosyl triazoles have rarely been tested as α-glucosidase inhibitors, partly due to inefficient synthesis of their precursor α-d-glucosylazide (αGA1). Glycosynthase enzymes, made by nucleophile mutations of retaining β-glucosidases, produce αGA1 in chemical rescue experiments. Thermoanaerobacterium xylanolyticus glucosyl hydrolase 116 β-glucosidase (TxGH116) E441G nucleophile mutant catalyzed synthesis of αGA1 from sodium azide and pNP-β-d-glucoside (pNPGlc) or cellobiose in aqueous medium at 45 °C. The pNPGlc and azide reaction product was purified by Sephadex LH-20 column chromatography to yield 280 mg of pure αGA1 (68% yield). αGA1 was successfully conjugated with alkynes attached to different functional groups, including aryl, ether, amine, amide, ester, alcohol, and flavone via copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry reactions. These reactions afforded the 1,4-substituted 1,2,3-triazole-α-d-glucoside derivatives AGT2-14 without protection and deprotection. Several of these glucosyl triazoles exhibited strong inhibition of human lysosomal α-glucosidase, with IC50 values for AGT4 and AGT14 more than 60-fold lower than that of the commercial α-glucosidase inhibitor acarbose.
中文翻译:

化学酶法和无保护基团合成对溶酶体 α-葡萄糖苷酶具有有效抑制活性的 1,4-取代 1,2,3-三唑-α-d-葡萄糖苷
α-葡萄糖基三唑很少被测试为 α-葡萄糖苷酶抑制剂,部分原因是其前体 α- d-葡萄糖基叠氮化物 ( αGA1 ) 的合成效率低下。糖合酶是由保留 β-葡萄糖苷酶的亲核突变产生的,在化学救援实验中产生αGA1 。热厌氧杆菌木糖基水解酶 116 β-葡萄糖苷酶 ( Tx GH116) E441G 亲核体突变体在 45 °C 水介质中催化从叠氮化钠和p NP-β- d-葡萄糖苷 ( p NPGlc) 或纤维二糖合成 αGA1。通过Sephadex LH-20柱色谱法纯化p NPGlc和叠氮化物反应产物,得到280mg纯αGA1 (产率68%)。通过铜催化的叠氮-炔环加成 (CuAAC) 点击化学反应, αGA1成功与连接到不同官能团的炔烃缀合,包括芳基、醚、胺、酰胺、酯、醇和黄酮。这些反应提供了没有保护和脱保护的1,4-取代的1,2,3-三唑-α- d-葡萄糖苷衍生物AGT2-14 。其中几种葡萄糖基三唑对人溶酶体 α-葡萄糖苷酶表现出强烈的抑制作用, AGT4和AGT14的 IC 50值比商业 α-葡萄糖苷酶抑制剂阿卡波糖低 60 倍以上。
更新日期:2021-10-06
中文翻译:

化学酶法和无保护基团合成对溶酶体 α-葡萄糖苷酶具有有效抑制活性的 1,4-取代 1,2,3-三唑-α-d-葡萄糖苷
α-葡萄糖基三唑很少被测试为 α-葡萄糖苷酶抑制剂,部分原因是其前体 α- d-葡萄糖基叠氮化物 ( αGA1 ) 的合成效率低下。糖合酶是由保留 β-葡萄糖苷酶的亲核突变产生的,在化学救援实验中产生αGA1 。热厌氧杆菌木糖基水解酶 116 β-葡萄糖苷酶 ( Tx GH116) E441G 亲核体突变体在 45 °C 水介质中催化从叠氮化钠和p NP-β- d-葡萄糖苷 ( p NPGlc) 或纤维二糖合成 αGA1。通过Sephadex LH-20柱色谱法纯化p NPGlc和叠氮化物反应产物,得到280mg纯αGA1 (产率68%)。通过铜催化的叠氮-炔环加成 (CuAAC) 点击化学反应, αGA1成功与连接到不同官能团的炔烃缀合,包括芳基、醚、胺、酰胺、酯、醇和黄酮。这些反应提供了没有保护和脱保护的1,4-取代的1,2,3-三唑-α- d-葡萄糖苷衍生物AGT2-14 。其中几种葡萄糖基三唑对人溶酶体 α-葡萄糖苷酶表现出强烈的抑制作用, AGT4和AGT14的 IC 50值比商业 α-葡萄糖苷酶抑制剂阿卡波糖低 60 倍以上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号