Chemical Data Collections Pub Date : 2021-09-23 , DOI: 10.1016/j.cdc.2021.100778 Hayat Ullah 1 , Imad Uddin 2 , Misbah 3 , Fahad Khan 4 , Muhammad Taha 5 , Fazal Rahim 4 , Maliha Sarfraz 6 , Sulaiman Shams 7 , Muhammad Nabi 8 , Abdul Wadood 7
|
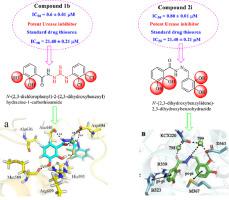
Benzohydrazide bearing thiourea/Schiff base analogs (1a-1k, 2a-2q) were synthesized and characterized through 1H, 13CNMR, HR-EIMS, FT-IR analysis. Urease inhibitory potential of all synthesized analogs were evaluated showing excellent inhibitory potential ranging between 0.6 ± 0.01 to 14.30 ± 0.02 and 0.80 ± 0.01 to 29.60 ± 1.00 μM respectively when compared with the standard drug thiourea (IC50 = 21.40 ± 0.21 μM). Analog 1b (IC50 = 0.6 ± 0.01 µM) was the most potent in benzohydrazide bearing thiourea series, while analog 2i (IC50 = 0.80 ± 0.01 μM) was the most potent in benzohydrazide bearing Schiff bases series. Our current study reveals new series of urease inhibitors for further analysis. Structure activity relationship was established to explore the effect of different substituent's attached to phenyl ring. Binding interactions of most potent analogs were established with help of docking studies.
中文翻译:

取代苯甲酰肼衍生物的合成:体外脲酶活性及其分子对接研究
合成含有硫脲/席夫碱类似物(1a - 1k、2a - 2q)的苯甲酰肼,并通过 1 H、 13 CNMR、HR-EIMS、FT-IR 分析对其进行表征。与标准药物硫脲 (IC 50 = 21.40 ± 0)相比,对所有合成类似物的脲酶抑制潜力进行了评估,显示出优异的抑制潜力,范围分别为 0.6 ± 0.01 至 14.30 ± 0.02 和 0.80 ± 0.01 至 29.60 ± 1.00 μM 。模拟1b (IC 50 = 0.6 ± 0.01 µM) 在含硫脲的苯甲酰肼系列中最有效,而模拟2i (IC 50 = 0.80 ± 0.01 μM) 在含苯甲酰肼的希夫碱系列中最有效。我们目前的研究揭示了用于进一步分析的新系列脲酶抑制剂。建立构效关系,探讨不同取代基对苯环的影响。大多数有效类似物的结合相互作用是在对接研究的帮助下建立的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号