Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards understanding of electrolyte degradation in lithium-mediated non-aqueous electrochemical ammonia synthesis with gas chromatography-mass spectrometry
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-23 , DOI: 10.1039/d1ra05963g Rokas Sažinas 1 , Suzanne Zamany Andersen 1 , Katja Li 1 , Mattia Saccoccio 1 , Kevin Krempl 1 , Jakob Bruun Pedersen 1 , Jakob Kibsgaard 1 , Peter Christian Kjærgaard Vesborg 1 , Debasish Chakraborty 1 , Ib Chorkendorff 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-23 , DOI: 10.1039/d1ra05963g Rokas Sažinas 1 , Suzanne Zamany Andersen 1 , Katja Li 1 , Mattia Saccoccio 1 , Kevin Krempl 1 , Jakob Bruun Pedersen 1 , Jakob Kibsgaard 1 , Peter Christian Kjærgaard Vesborg 1 , Debasish Chakraborty 1 , Ib Chorkendorff 1
Affiliation
|
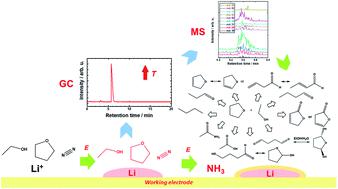
Lithium-mediated electrochemical ammonia synthesis (LiMEAS) in non-aqueous media is a promising technique for efficient and green ammonia synthesis. Compared to the widely used Haber–Bosch process, the method reduces CO2 emissions to zero due to the application of green hydrogen. However, the non-aqueous medium encounters the alkali metal lithium and organic components at high negative potentials of electrolysis, which leads to formation of byproducts. To assess the environmental risk of this synthesis method, standardized analytical methods towards understanding of the degradation level and consequences are needed. Here we report on the implementation of an approach to analyze the liquid electrolytes after electrochemical ammonia synthesis via high-resolution gas chromatography-mass spectrometry (GCMS). To characterize the molecular species formed after electrolysis, electron ionization high-resolution mass spectrometry (EI-MS) was applied. The fragmentation patterns enabled the elucidation of the mechanisms of byproduct formation. Several organic electrolytes were analyzed and compared both qualitatively and quantitatively to ascertain molecular composition and degradation products. It was found that the organic solvent in contact with metallic electrodeposited lithium induces solvent degradation, and the extent of this decomposition to different organic molecules depends on the organic solvent used. Our results show GCMS as a suitable technique for monitoring non-aqueous electrochemical ammonia synthesis in different organic electrolytes.
中文翻译:

用气相色谱-质谱法理解锂介导的非水电化学氨合成中的电解质降解
在非水介质中锂介导的电化学氨合成(LiMEAS)是一种有前途的高效绿色合成氨技术。与广泛使用的 Haber-Bosch 工艺相比,该方法由于使用了绿色氢气,将 CO 2排放量降至零。然而,非水介质在电解的高负电位下遇到碱金属锂和有机成分,从而导致副产物的形成。为了评估这种合成方法的环境风险,需要标准化的分析方法来了解降解水平和后果。在这里,我们报告了一种分析电化学氨合成后液体电解质的方法的实施情况。高分辨率气相色谱-质谱 (GCMS)。为了表征电解后形成的分子种类,应用了电子电离高分辨率质谱 (EI-MS)。碎片模式能够阐明副产物形成的机制。对几种有机电解质进行了定性和定量分析和比较,以确定分子组成和降解产物。发现与金属电沉积锂接触的有机溶剂会引起溶剂降解,这种分解成不同有机分子的程度取决于所使用的有机溶剂。我们的结果表明 GCMS 作为监测不同有机电解质中非水电化学氨合成的合适技术。
更新日期:2021-09-23
中文翻译:

用气相色谱-质谱法理解锂介导的非水电化学氨合成中的电解质降解
在非水介质中锂介导的电化学氨合成(LiMEAS)是一种有前途的高效绿色合成氨技术。与广泛使用的 Haber-Bosch 工艺相比,该方法由于使用了绿色氢气,将 CO 2排放量降至零。然而,非水介质在电解的高负电位下遇到碱金属锂和有机成分,从而导致副产物的形成。为了评估这种合成方法的环境风险,需要标准化的分析方法来了解降解水平和后果。在这里,我们报告了一种分析电化学氨合成后液体电解质的方法的实施情况。高分辨率气相色谱-质谱 (GCMS)。为了表征电解后形成的分子种类,应用了电子电离高分辨率质谱 (EI-MS)。碎片模式能够阐明副产物形成的机制。对几种有机电解质进行了定性和定量分析和比较,以确定分子组成和降解产物。发现与金属电沉积锂接触的有机溶剂会引起溶剂降解,这种分解成不同有机分子的程度取决于所使用的有机溶剂。我们的结果表明 GCMS 作为监测不同有机电解质中非水电化学氨合成的合适技术。











































 京公网安备 11010802027423号
京公网安备 11010802027423号