当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane-Bound Flavocytochrome MsrQ Is a Substrate of the Flavin Reductase Fre in Escherichia coli
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-22 , DOI: 10.1021/acschembio.1c00613 Christelle Caux 1 , Bruno Guigliarelli 2 , Corinne Vivès 3 , Frédéric Biaso 2 , Marius Horeau 1 , Hawra Hassoune 1 , Isabelle Petit-Hartlein 3 , Céline Juillan-Binard 3 , Stephane Torelli 1 , Franck Fieschi 3 , Vincent Nivière 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-22 , DOI: 10.1021/acschembio.1c00613 Christelle Caux 1 , Bruno Guigliarelli 2 , Corinne Vivès 3 , Frédéric Biaso 2 , Marius Horeau 1 , Hawra Hassoune 1 , Isabelle Petit-Hartlein 3 , Céline Juillan-Binard 3 , Stephane Torelli 1 , Franck Fieschi 3 , Vincent Nivière 1
Affiliation
|
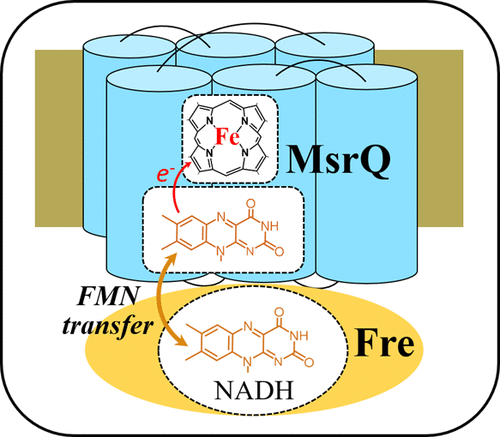
MsrPQ is a new type of methionine sulfoxide reductase (Msr) system found in bacteria. It is specifically involved in the repair of periplasmic methionine residues that are oxidized by hypochlorous acid. MsrP is a periplasmic molybdoenzyme that carries out the Msr activity, whereas MsrQ, an integral membrane-bound hemoprotein, acts as the physiological partner of MsrP to provide electrons for catalysis. Although MsrQ (YedZ) was associated since long with a protein superfamily named FRD (ferric reductase domain), including the eukaryotic NADPH oxidases and STEAP proteins, its biochemical properties are still sparsely documented. Here, we have investigated the cofactor content of the E. coli MsrQ and its mechanism of reduction by the flavin reductase Fre. We showed by electron paramagnetic resonance (EPR) spectroscopy that MsrQ contains a single highly anisotropic low-spin (HALS) b-type heme located on the periplasmic side of the membrane. We further demonstrated that MsrQ holds a flavin mononucleotide (FMN) cofactor that occupies the site where a second heme binds in other members of the FDR superfamily on the cytosolic side of the membrane. EPR spectroscopy indicates that the FMN cofactor can accommodate a radical semiquinone species. The cytosolic flavin reductase Fre was previously shown to reduce the MsrQ heme. Here, we demonstrated that Fre uses the FMN MsrQ cofactor as a substrate to catalyze the electron transfer from cytosolic NADH to the heme. Formation of a specific complex between MsrQ and Fre could favor this unprecedented mechanism, which most likely involves transfer of the reduced FMN cofactor from the Fre active site to MsrQ.
中文翻译:

膜结合黄素细胞色素 MsrQ 是大肠杆菌中黄素还原酶 Fre 的底物
MsrPQ 是一种在细菌中发现的新型蛋氨酸亚砜还原酶 (Msr) 系统。它特别参与修复被次氯酸氧化的周质蛋氨酸残基。MsrP 是一种执行 Msr 活性的周质钼酶,而 MsrQ 是一种完整的膜结合血蛋白,作为 MsrP 的生理伙伴,为催化提供电子。尽管 MsrQ (YedZ) 长期以来与名为 FRD(铁还原酶结构域)的蛋白质超家族相关,包括真核 NADPH 氧化酶和 STEAP 蛋白,但其生化特性仍然很少被记录。在这里,我们研究了大肠杆菌的辅因子含量MsrQ 及其被黄素还原酶 Fre 还原的机制。我们通过电子顺磁共振 (EPR) 光谱表明 MsrQ 包含位于膜周质侧的单个高度各向异性低自旋 (HALS) b 型血红素。我们进一步证明,MsrQ 拥有一个黄素单核苷酸 (FMN) 辅因子,该辅因子占据了第二个血红素与 FDR 超家族其他成员在细胞溶质侧结合的位点。EPR 光谱表明 FMN 辅因子可以容纳自由基半醌物种。细胞溶质黄素还原酶 Fre 先前被证明可以减少 MsrQ 血红素。在这里,我们证明了 Fre 使用 FMN MsrQ 辅因子作为底物来催化从细胞溶质 NADH 到血红素的电子转移。
更新日期:2021-11-19
中文翻译:

膜结合黄素细胞色素 MsrQ 是大肠杆菌中黄素还原酶 Fre 的底物
MsrPQ 是一种在细菌中发现的新型蛋氨酸亚砜还原酶 (Msr) 系统。它特别参与修复被次氯酸氧化的周质蛋氨酸残基。MsrP 是一种执行 Msr 活性的周质钼酶,而 MsrQ 是一种完整的膜结合血蛋白,作为 MsrP 的生理伙伴,为催化提供电子。尽管 MsrQ (YedZ) 长期以来与名为 FRD(铁还原酶结构域)的蛋白质超家族相关,包括真核 NADPH 氧化酶和 STEAP 蛋白,但其生化特性仍然很少被记录。在这里,我们研究了大肠杆菌的辅因子含量MsrQ 及其被黄素还原酶 Fre 还原的机制。我们通过电子顺磁共振 (EPR) 光谱表明 MsrQ 包含位于膜周质侧的单个高度各向异性低自旋 (HALS) b 型血红素。我们进一步证明,MsrQ 拥有一个黄素单核苷酸 (FMN) 辅因子,该辅因子占据了第二个血红素与 FDR 超家族其他成员在细胞溶质侧结合的位点。EPR 光谱表明 FMN 辅因子可以容纳自由基半醌物种。细胞溶质黄素还原酶 Fre 先前被证明可以减少 MsrQ 血红素。在这里,我们证明了 Fre 使用 FMN MsrQ 辅因子作为底物来催化从细胞溶质 NADH 到血红素的电子转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号