Water Research ( IF 11.4 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.watres.2021.117684 Lin Wang 1 , Tingting Yan 2 , Ruijie Tang 2 , Qian Ping 1 , Yongmei Li 1 , Jie Wang 3
|
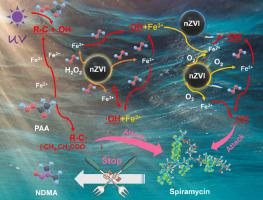
In this study, nanoscale zero-valent iron (nZVI) was added to motivate the functions of all the reactive oxidation species in peracetic acid (PAA) mixture under ultraviolet (UV) irradiation, and to enhance the removal of spiramycin, which is a typical precursor of N-nitrosodimethylamine (NDMA). Spiramycin (≤ 10 mg/L) could be completely removed within 20 min under the conditions of an initial pH of 4.0, a nZVI dose of 0.02 g/L and a PAA dose of 3.0 mg/L; additionally, 95.8% and 78.8% of PAA and H2O2 were consumed during the process. Electron paramagnetic resonance analysis and quenching experiments confirmed that 52.4% and 44.8% of spiramycin removal was contributed by hydroxyl radical (•OH) and carbon-centered radicals (R-C•), respectively; and Fe2+ released from nZVI played a critical role in radicals generation. Four degradation pathways of spiramycin were proposed and verified by the density of functional theory analysis. 65.2% of the NDMA formation potential (FP) was reduced after the reaction, and its residual was mainly contributed by the undegraded intermediate of dimethylamine. The results of multiple characterizations and continuous degradation experiments indicated that nZVI was stable in the system as the removal of spiramycin was hardly influenced even if reused three times. The nZVI/UV/PAA process is a promising advanced oxidation technology not only for the removal of refractory NDMA precursors (such as spiramycin) but also for significantly lowering the NDMA FP.
中文翻译:

通过添加纳米零价铁在紫外线照射下协同去除螺旋霉素对过乙酸中反应性氧化物质的促进作用:机制和 N-亚硝基二甲胺形成潜力评估
在这项研究中,添加纳米级零价铁 (nZVI) 以激发过乙酸 (PAA) 混合物中所有活性氧化物质在紫外线 (UV) 照射下的功能,并增强螺旋霉素的去除,这是典型的螺旋霉素。 N-亚硝基二甲胺 (NDMA) 的前体。在初始pH为4.0、nZVI剂量为0.02 g/L、PAA剂量为3.0 mg/L的条件下,螺旋霉素(≤10 mg/L)可在20分钟内完全去除;此外,在该过程中消耗了95.8%和78.8%的PAA和H 2 O 2。电子顺磁共振分析和猝灭实验证实,52.4% 和 44.8% 的螺旋霉素去除率分别来自羟基自由基 (•OH) 和碳中心自由基 (RC•);和 Fe 2+nZVI 释放的自由基在自由基生成中起关键作用。通过密度泛函理论分析,提出并验证了螺旋霉素的四种降解途径。反应后65.2%的NDMA形成电位(FP)降低,其残留主要由未降解的二甲胺中间体贡献。多次表征和连续降解实验结果表明,nZVI在体系中稳定,即使重复使用3次,对螺旋霉素的去除也几乎没有影响。nZVI/UV/PAA 工艺是一种很有前途的高级氧化技术,不仅可以去除难降解的 NDMA 前体(如螺旋霉素),还可以显着降低 NDMA FP。









































 京公网安备 11010802027423号
京公网安备 11010802027423号