Veterinary Microbiology ( IF 2.4 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.vetmic.2021.109242 Jinli Wang 1 , Jinyue Zhu 1 , Jinwu Meng 1 , Tianxin Qiu 1 , Wenjia Wang 1 , Rui Wang 1 , Jiaguo Liu 1
|
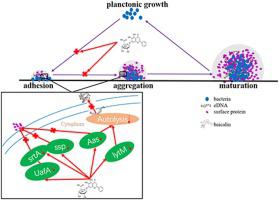
The ability to form biofilms on surfaces makes Staphylococcus saprophyticus (S. saprophyticus) becomes the main pathogenic factor in nosocomial infections. Previously, we demonstrated that baicalin (Bac) inhibited azithromycin-resistant S. saprophyticus (ARSS) biofilm formation. This investigation aims to explore the influence of baicalin on primary adhesion and aggregation phases of biofilm formation, and the treatment effect of baicalin and azithromycin on ARSS biofilm-associated infection. Crystal violet (CV) staining and scanning electron microscope (SEM) observations clearly showed that sub-inhibitory concentration baicalin inhibited ARSS biofilm formation when baicalin was added before the adhesion and aggregation phases. Baicalin significantly increased the relative adhesion inhibition rate and decreased the rate of bacteria aggregation in a dose-dependent manner. Moreover, CLSM and cell lysis assays revealed that baicalin inhibited the production of surface proteins and cell autolysis in bacteria adhesion and aggregation phases of biofilm formation. Meanwhile, the relative expressions of adhesion-related and autolysis-related genes were down-regulated by baicalin. In vivo, the combination of baicalin and azithromycin succeeded in eradicating ARSS from the mouse cutaneous infection model and decreasing the pathological injuries, the expressions of cytokines in infected tissue, and the number of inflammatory cells in the blood. Simultaneously, baicalin decreased the bacterial burdens in tubes, the level of TNF-α, and the number of monocytes and neutrophils compared with that of the SS and azithromycin groups. Based on these results, baicalin inhibited the adhesion and aggregation phases of biofilm formation by influenced the production of surface proteins and cell autolysis. Baicalin and azithromycin synergetically treated ARSS biofilm-associated infection.
中文翻译:

黄芩苷通过影响腐生葡萄球菌的初级粘附和聚集相抑制生物膜形成
在表面形成生物膜的能力使得腐生葡萄球菌(S. saprophyticus)成为医院感染的主要致病因素。此前,我们证明黄芩苷 (Bac) 抑制了耐阿奇霉素的腐生链球菌(ARSS) 生物膜形成。本研究旨在探讨黄芩苷对生物膜形成初期粘附和聚集阶段的影响,以及黄芩苷和阿奇霉素对ARSS生物膜相关感染的治疗效果。结晶紫 (CV) 染色和扫描电子显微镜 (SEM) 观察清楚地表明,当在粘附和聚集阶段之前加入黄芩苷时,亚抑制浓度的黄芩苷抑制了 ARSS 生物膜的形成。黄芩苷以剂量依赖性方式显着增加相对粘附抑制率并降低细菌聚集率。此外,CLSM 和细胞裂解试验表明,黄芩苷在细菌粘附和生物膜形成的聚集阶段抑制表面蛋白的产生和细胞自溶。同时,黄芩苷下调黏附相关基因和自溶相关基因的相对表达。在体内,黄芩苷和阿奇霉素的组合成功地从小鼠皮肤感染模型中根除 ARSS,并减少了病理损伤、感染组织中细胞因子的表达以及血液中炎症细胞的数量。同时,与 SS 和阿奇霉素组相比,黄芩苷降低了管内细菌负荷、TNF-α 水平以及单核细胞和中性粒细胞的数量。基于这些结果,黄芩苷通过影响表面蛋白的产生和细胞自溶来抑制生物膜形成的粘附和聚集阶段。黄芩苷和阿奇霉素协同治疗 ARSS 生物膜相关感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号