当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Chiral Resolution of Twisted Carbon Nanobelts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-22 , DOI: 10.1021/jacs.1c08468 Wei Fan 1 , Taisuke Matsuno 2 , Yi Han 1 , Xuhui Wang 1 , Qifeng Zhou 1, 3 , Hiroyuki Isobe 2 , Jishan Wu 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-22 , DOI: 10.1021/jacs.1c08468 Wei Fan 1 , Taisuke Matsuno 2 , Yi Han 1 , Xuhui Wang 1 , Qifeng Zhou 1, 3 , Hiroyuki Isobe 2 , Jishan Wu 1, 3
Affiliation
|
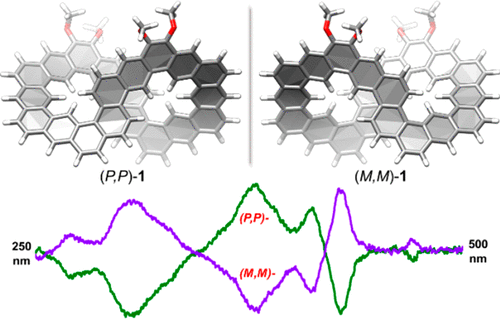
Twisted carbon nanobelts could display persistent chirality, which is desirable for material applications, but their synthesis is very challenging. Herein, we report the successful synthesis and chiral resolution of such a kind of molecules (1-H and 1) with a figure-eight configuration. 1-H was synthesized first by macrocyclization through Suzuki coupling reaction followed by benzannulation via Bi(OTf)3-mediated cyclization reaction of vinyl ether. Oxidative dehydrogenation of 1-H gave the fully π-conjugated 1. Their twisted structures were confirmed by X-ray crystallographic analysis and calculations, and they can be resolved by chiral high-performance liquid chromatography. The isolated enantiomers showed persistent chiroptical properties according to the circular dichroism measurements, with moderate |gabs| values (0.0016 for 1-H and 0.005–0.007 for 1). Their photophysical properties were also briefly studied.
中文翻译:

扭曲碳纳米带的合成和手性分离
扭曲的碳纳米带可以显示出持久的手性,这对于材料应用来说是理想的,但它们的合成非常具有挑战性。在此,我们报告了这种具有八字形构型的分子(1-H和1)的成功合成和手性拆分。首先通过Suzuki偶联反应通过大环化合成1-H,然后通过Bi(OTf) 3介导的乙烯基醚环化反应进行苯环化。1-H 的氧化脱氢得到完全 π 共轭的1. 它们的扭曲结构通过 X 射线晶体学分析和计算得到证实,并且可以通过手性高效液相色谱分离。根据圆二色性测量,分离的对映异构体显示出持久的手性特性,中等 | g绝对值| 值(0.0016为1-H和0.005-0.007为1)。还简要研究了它们的光物理性质。
更新日期:2021-10-06
中文翻译:

扭曲碳纳米带的合成和手性分离
扭曲的碳纳米带可以显示出持久的手性,这对于材料应用来说是理想的,但它们的合成非常具有挑战性。在此,我们报告了这种具有八字形构型的分子(1-H和1)的成功合成和手性拆分。首先通过Suzuki偶联反应通过大环化合成1-H,然后通过Bi(OTf) 3介导的乙烯基醚环化反应进行苯环化。1-H 的氧化脱氢得到完全 π 共轭的1. 它们的扭曲结构通过 X 射线晶体学分析和计算得到证实,并且可以通过手性高效液相色谱分离。根据圆二色性测量,分离的对映异构体显示出持久的手性特性,中等 | g绝对值| 值(0.0016为1-H和0.005-0.007为1)。还简要研究了它们的光物理性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号