当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Iso(thio)cyanates with Dialanes: Cycloaddition, Reductive Coupling, or Cleavage of the C═S or C═O Bond
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.inorgchem.1c01581 Vladimir A Dodonov 1 , Weixing Chen 2 , Li Liu 2 , Vladimir G Sokolov 1 , Evgeny V Baranov 1 , Alexandra A Skatova 1 , Yanxia Zhao 2 , Biao Wu 2 , Xiao-Juan Yang 2 , Igor L Fedushkin 1, 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.inorgchem.1c01581 Vladimir A Dodonov 1 , Weixing Chen 2 , Li Liu 2 , Vladimir G Sokolov 1 , Evgeny V Baranov 1 , Alexandra A Skatova 1 , Yanxia Zhao 2 , Biao Wu 2 , Xiao-Juan Yang 2 , Igor L Fedushkin 1, 2
Affiliation
|
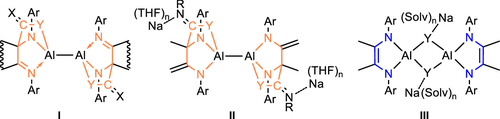
The dialanes [(dpp-Bian)Al–Al(dpp-Bian)] (1) and [(dpp-dad)Al(THF)–(THF)Al(dpp-dad)] (2) (dpp-Bian = 1,2-[(2,6-iPr2C6H3)NC]2C12H6, dpp-dad = [(2,6-iPr2C6H3)NC(CH3)]2) react with some isothiocyanates, isocyanates, and diphenylketene via [2 + 4] cycloaddition of the C═O or C═S bond across the C═C—N—Al fragment to afford complexes [L(X═C—Y)Al—Al(X═C—Y)L] with an intact Al–Al single bond (3, L = dpp-Bian, X = PhN, Y = O; 4, L = dpp-Bian, X = Ph2C, Y = O; 6, L = dpp-dad, X = BnN, Y = S; 7, L = dpp-dad, X = tBuN, Y = O; 8, L = dpp-dad, X = iPrN, Y = S; and 9, L = dpp-dad, X = CyN, Y = S). A mixed C═N and C═O mode cycloadduct, [(dpp-Bian)(TosN═C—O)Al—Al(TosN—C═O)(dpp-Bian)] 5, was obtained in the reaction of 1 with tosylisocyanate. Heating the solution of 3 resulted in a thermal transformation and a change of the cycloaddition mode from C═O to C═N to give the product [(dpp-Bian)(PhN—C═O)Al(O)Al(PhN—C═O)(dpp-Bian)] 10. The reduction of 7 and 8 with Na yielded the products [Na(THF)n]2[(dpp-dad–H)(X═C—Y)Al]2 (12, X = iPrN, Y = S, n = 2 and 13, X = tBuN, Y = O, n = 3) in which one of the methyl groups of the backbone of the initial dpp-dad ligand was dehydrogenated. When 2 was reacted with the bulky adamantyl isocyanate AdNCO, the C–C coupling of two substrates occurred to form 14 [(dpp-dad)Al(O═C—NAd)2Al(dpp-dad)] in which the coupled dianionic oxamide ligand bridged two Al atoms in a μ,η4-N,O/N,O mode. Moreover, in the presence of 2.0 equiv of Na metal, precursor 2 reacts with tBuNCS, p-TolylNCS, or Me3SiNCO, possibly through the reduced AlI intermediate, to yield the sulfur- or oxygen-bridged dimer [Na(solv)n]2[(dpp-dad)Al(μ-E)]2 (15, E = S, solv = THF, n = 3 and 16, E = O, solv = DME, n = 2) upon C═S or C═O bond cleavage. Dialane 1 reacts with dimethylsulfone to give a Lewis adduct [(dpp-Bian)(Me2SO2)Al]2 (17), which releases dimethylsulfone upon heating. The diamagnetic compounds 3–10 and 12–17 were characterized by NMR and IR spectroscopy. The molecular structures of 3–17 were established by single-crystal X-ray diffraction analysis. Electronic structures of the compounds and possible isomers have been examined by DFT calculations.
中文翻译:

异(硫)氰酸酯与二烷的反应:C=S 或 C=O 键的环加成、还原偶联或裂解
[(dpp-Bian)Al-Al(dpp-Bian)] ( 1 ) 和 [(dpp-dad)Al(THF)-(THF)Al(dpp-dad)] ( 2 ) (dpp-Bian = 1,2-[( 2,6 - i Pr 2 C 6 H 3 )NC] 2 C 12 H 6 , dpp-dad = [( 2,6 - i Pr 2 C 6 H 3 )NC(CH 3 )] 2 ) 通过跨 C=CN-Al 片段的 C=O 或 C=S 键的 [2 + 4] 环加成与一些异硫氰酸酯、异氰酸酯和二苯基乙烯酮反应,得到复合物 [L(X=C-Y) Al-Al( X=C -Y)L] 具有完整的 Al-Al 单键 ( 3 , L = dpp-Bian, X = PhN, Y = O;4 , L = dpp-Bian, X = Ph 2 C, Y = O; 6 , L = dpp-dad, X = BnN, Y = S; 7 , L = dpp-dad, X = t BuN, Y = O; 8 , L = dpp-dad, X = i PrN, Y = S; 和9,L = dpp-dad,X = CyN,Y = S)。混合C = N和C = O模式环加成,[(DPP-扁)(TosN═C-O)铝铝(TosN-C = O)(DPP-扁)] 5中的反应得到,1与甲苯磺酰异氰酸酯。加热3的溶液导致热转变和环加成模式从 C=O 到 C=N 的变化,得到产物 [(dpp-Bian)(PhN-C=O)Al(O)Al(PhN- C=O)(dpp-Bian)] 10 . 减少7和8与 Na 产生产物 [Na(THF) n ] 2 [(dpp-dad –H )( X= C-Y)Al] 2 ( 12 , X = i PrN, Y = S, n = 2 和13 , X = t BuN, Y = O, n = 3) 其中初始 dpp-dad 配体骨架的甲基之一被脱氢。当2与庞大的异氰酸金刚烷基酯 AdNCO 反应时,两种底物的 C-C 偶联发生形成14 [(dpp-dad)Al(O=C-NAd) 2 Al(dpp-dad)],其中偶联的双阴离子草酰胺配体在 μ,n 4 中桥接两个 Al 原子-N,O/N,O 模式。此外,在 2.0 当量的 Na 金属存在下,前体2与t BuNCS、p- TolylNCS 或 Me 3 SiNCO 反应,可能通过还原的 Al I中间体,产生硫桥连或氧桥连的二聚体 [Na(solv ) n ] 2 [(dpp-dad)Al(μ-E)] 2 ( 15 , E = S, solv = THF, n = 3 and 16 , E = O, solv = DME, n = 2) on C= S 或 C=O 键断裂。Dialane 1与二甲基砜反应生成路易斯加合物 [(dpp-Bian)(Me 2 SO 2 )Al] 2 (17 ),加热时会释放二甲基砜。抗磁性化合物3 – 10和12 – 17通过核磁共振和红外光谱表征。的分子结构3 - 17由单晶X射线衍射分析来建立。已通过 DFT 计算检查了化合物和可能的异构体的电子结构。
更新日期:2021-10-04
中文翻译:

异(硫)氰酸酯与二烷的反应:C=S 或 C=O 键的环加成、还原偶联或裂解
[(dpp-Bian)Al-Al(dpp-Bian)] ( 1 ) 和 [(dpp-dad)Al(THF)-(THF)Al(dpp-dad)] ( 2 ) (dpp-Bian = 1,2-[( 2,6 - i Pr 2 C 6 H 3 )NC] 2 C 12 H 6 , dpp-dad = [( 2,6 - i Pr 2 C 6 H 3 )NC(CH 3 )] 2 ) 通过跨 C=CN-Al 片段的 C=O 或 C=S 键的 [2 + 4] 环加成与一些异硫氰酸酯、异氰酸酯和二苯基乙烯酮反应,得到复合物 [L(X=C-Y) Al-Al( X=C -Y)L] 具有完整的 Al-Al 单键 ( 3 , L = dpp-Bian, X = PhN, Y = O;4 , L = dpp-Bian, X = Ph 2 C, Y = O; 6 , L = dpp-dad, X = BnN, Y = S; 7 , L = dpp-dad, X = t BuN, Y = O; 8 , L = dpp-dad, X = i PrN, Y = S; 和9,L = dpp-dad,X = CyN,Y = S)。混合C = N和C = O模式环加成,[(DPP-扁)(TosN═C-O)铝铝(TosN-C = O)(DPP-扁)] 5中的反应得到,1与甲苯磺酰异氰酸酯。加热3的溶液导致热转变和环加成模式从 C=O 到 C=N 的变化,得到产物 [(dpp-Bian)(PhN-C=O)Al(O)Al(PhN- C=O)(dpp-Bian)] 10 . 减少7和8与 Na 产生产物 [Na(THF) n ] 2 [(dpp-dad –H )( X= C-Y)Al] 2 ( 12 , X = i PrN, Y = S, n = 2 和13 , X = t BuN, Y = O, n = 3) 其中初始 dpp-dad 配体骨架的甲基之一被脱氢。当2与庞大的异氰酸金刚烷基酯 AdNCO 反应时,两种底物的 C-C 偶联发生形成14 [(dpp-dad)Al(O=C-NAd) 2 Al(dpp-dad)],其中偶联的双阴离子草酰胺配体在 μ,n 4 中桥接两个 Al 原子-N,O/N,O 模式。此外,在 2.0 当量的 Na 金属存在下,前体2与t BuNCS、p- TolylNCS 或 Me 3 SiNCO 反应,可能通过还原的 Al I中间体,产生硫桥连或氧桥连的二聚体 [Na(solv ) n ] 2 [(dpp-dad)Al(μ-E)] 2 ( 15 , E = S, solv = THF, n = 3 and 16 , E = O, solv = DME, n = 2) on C= S 或 C=O 键断裂。Dialane 1与二甲基砜反应生成路易斯加合物 [(dpp-Bian)(Me 2 SO 2 )Al] 2 (17 ),加热时会释放二甲基砜。抗磁性化合物3 – 10和12 – 17通过核磁共振和红外光谱表征。的分子结构3 - 17由单晶X射线衍射分析来建立。已通过 DFT 计算检查了化合物和可能的异构体的电子结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号