当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Thermodynamics of Eu(III) and Cm(III) Complexes with Glucuronic Acid
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.inorgchem.1c01746 Sebastian Reese 1 , Peter Kaden 1 , Corey J Taylor 1 , Roger Kloditz 1 , Moritz Schmidt 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.inorgchem.1c01746 Sebastian Reese 1 , Peter Kaden 1 , Corey J Taylor 1 , Roger Kloditz 1 , Moritz Schmidt 1
Affiliation
|
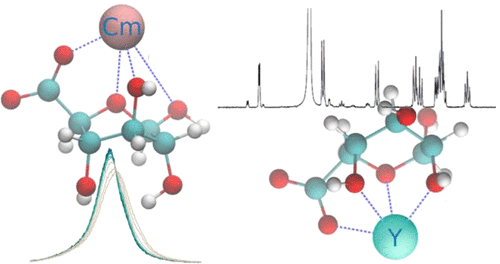
Complexation by small organic ligands controls the bioavailability of contaminants and influences their mobility in the geosphere. We have studied the interactions of Cm3+, as a representative of the trivalent actinides, and Eu3+, as an inactive homologue, with glucuronic acid (GlcA) a simple sugar acid. Time-resolved laser-induced luminescence spectroscopy (TRLFS) shows that complexation at pH 5.0 occurs only at high ligand to metal ratios in the form of 1:1 complexes with standard formation constants log β0 = 1.84 ± 0.22 for Eu3+ and log β0 = 2.39 ± 0.19 for Cm3+. A combination of NMR, QMMM, and TRLFS reveals the structure of the complex to be a half-sandwich structure wherein the ligand binds through its carboxylic group, the ring oxygen, and a hydroxyl group in addition to five to six water molecules. Surprisingly, Y3+, which was used as a diamagnetic reference in NMR, prefers a different coordination geometry with bonding through at least two hydroxyl groups on the opposite side of a distorted GlcA molecule. QMMM simulations indicate that the differences in stability among Cm, Eu, and Y are related to ring strain induced by smaller cations. At higher pH a stronger complex was detected, most likely due to deprotonation of a coordinating OH group.
中文翻译:

Eu(III) 和 Cm(III) 与葡萄糖醛酸配合物的结构和热力学
小有机配体的络合控制污染物的生物利用度并影响它们在地圈中的流动性。我们研究了作为三价锕系元素代表的 Cm 3+和作为无活性同系物的Eu 3+与葡萄糖醛酸 (GlcA) 一种单糖酸的相互作用。时间分辨激光诱导发光光谱 (TRLFS) 表明,pH 5.0 下的络合仅在配体与金属的高比例下以 1:1 络合物的形式发生,Eu 3+和 log 的标准形成常数 log β 0 = 1.84 ± 0.22对于 Cm 3+, β 0 = 2.39 ± 0.19. NMR、QMMM 和 TRLFS 的组合揭示了该复合物的结构为半夹心结构,其中配体通过其羧基、环氧和羟基以及五到六个水分子结合。令人惊讶的是,在 NMR 中用作抗磁性参考的Y 3+更喜欢不同的配位几何结构,通过扭曲的 GlcA 分子的另一侧的至少两个羟基键合。QMMM 模拟表明,Cm、Eu 和 Y 之间稳定性的差异与较小阳离子引起的环应变有关。在较高的 pH 值下,检测到更强的复合物,很可能是由于配位 OH 基团的去质子化。
更新日期:2021-10-04
中文翻译:

Eu(III) 和 Cm(III) 与葡萄糖醛酸配合物的结构和热力学
小有机配体的络合控制污染物的生物利用度并影响它们在地圈中的流动性。我们研究了作为三价锕系元素代表的 Cm 3+和作为无活性同系物的Eu 3+与葡萄糖醛酸 (GlcA) 一种单糖酸的相互作用。时间分辨激光诱导发光光谱 (TRLFS) 表明,pH 5.0 下的络合仅在配体与金属的高比例下以 1:1 络合物的形式发生,Eu 3+和 log 的标准形成常数 log β 0 = 1.84 ± 0.22对于 Cm 3+, β 0 = 2.39 ± 0.19. NMR、QMMM 和 TRLFS 的组合揭示了该复合物的结构为半夹心结构,其中配体通过其羧基、环氧和羟基以及五到六个水分子结合。令人惊讶的是,在 NMR 中用作抗磁性参考的Y 3+更喜欢不同的配位几何结构,通过扭曲的 GlcA 分子的另一侧的至少两个羟基键合。QMMM 模拟表明,Cm、Eu 和 Y 之间稳定性的差异与较小阳离子引起的环应变有关。在较高的 pH 值下,检测到更强的复合物,很可能是由于配位 OH 基团的去质子化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号