Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.colsurfb.2021.112125 Peng Zhang 1 , Xinfang Li 1 , Qinan Xu 1 , Youxiang Wang 1 , Jian Ji 1
|
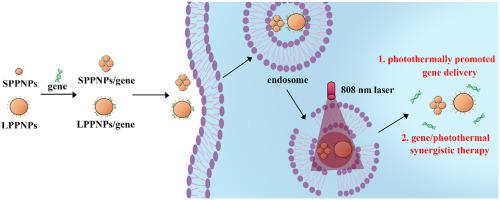
The combination of photothermal therapy and gene therapy has received increasing attention in tumor treatment. However, how to improve synergistic efficacy has become a new challenge. NIR light has a great potential in tumor treatment because of its considerable penetration depth and spatiotemporal controllability. Polydopamine is a popular photothermal conversion agent, which has desirable photothermal conversion ability and good biocompatibility. In this research, polydopamine-polyethyleneimine nanoparticles with diameters of 13 nm (SPPNPs) and 236 nm (LPPNPs) were prepared as gene carriers. The size of polydopamine nanoparticles had great effect on the complexes formation, photothermal conversion ability and gene transfection efficiency. After loading gene, the SPPNPs/gene and LPPNPs/gene complexes were about 60–80 nm and 240 nm respectively, indicating different styles of complexes formation. Both SPPNPs/gene and LPPNPs/gene complexes without NIR irradiation could achieve similar gene transfection efficiency as commercial lipofectamine 2000, while with lower cytotoxicity. Due to better photothermal conversion ability, the transfection level of LPPNPs/pGL-3 complexes increased to 4.5 times after NIR irradiation (2.6 W/cm2, 15 min), which ascribed to the quick escape of gene complexes from the endosome. The produced heat under NIR irradiation could also ablate tumor cells. So LPPNPs were chosen to deliver tumor suppressor gene p53 DNA to investigate the synergistic efficacy of gene/photothermal therapy. The tumor in KB tumor-bearing mice was almost eliminated after intratumoral injection, and the tumor inhibition efficacy of gene/photothermal synergistic therapy achieved to 99%. By combining NIR-promoted gene transfection and gene/photothermal synergistic therapy, the LPPNPs hold great promise in practical tumor treatment.
中文翻译:

用于近红外促进基因传递和协同光热治疗的不同大小的聚多巴胺纳米粒子
光热疗法和基因疗法的结合在肿瘤治疗中越来越受到重视。然而,如何提高协同效应成为新的挑战。近红外光由于具有相当大的穿透深度和时空可控性,在肿瘤治疗中具有巨大的潜力。聚多巴胺是一种流行的光热转化剂,具有良好的光热转化能力和良好的生物相容性。在这项研究中,制备了直径为 13 nm (SPPNPs) 和 236 nm (LPPNPs) 的聚多巴胺-聚乙烯亚胺纳米粒子作为基因载体。聚多巴胺纳米粒子的大小对复合物的形成、光热转换能力和基因转染效率有很大影响。加载基因后,SPPNPs/基因和 LPPNPs/基因复合物分别约为 60-80 nm 和 240 nm,表示不同风格的配合物形成。没有 NIR 照射的 SPPNPs/基因和 LPPNPs/基因复合物都可以达到与商业 lipofectamine 2000 相似的基因转染效率,同时具有较低的细胞毒性。由于更好的光热转换能力,LPPNPs/pGL-3 复合物的转染水平在 NIR 照射后提高到 4.5 倍(2.6 W/cm2 , 15 分钟),这归因于基因复合物从内体快速逃逸。NIR 照射下产生的热量也可以消融肿瘤细胞。因此选择LPPNPs传递抑癌基因p53 DNA来研究基因/光热疗法的协同功效。KB荷瘤小鼠瘤内注射后肿瘤几乎消失,基因/光热协同治疗的抑瘤效果达到99%。通过将 NIR 促进的基因转染和基因/光热协同治疗相结合,LPPNPs 在实际的肿瘤治疗中具有很大的应用前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号