Chemosphere ( IF 8.1 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.chemosphere.2021.132286 Mohd Azfar Shaida 1 , R K Dutta 1 , A K Sen 2 , S S Ram 3 , M Sudarshan 3 , Mu Naushad 4 , Grzegorz Boczkaj 5 , Md Sadique Nawab 6
|
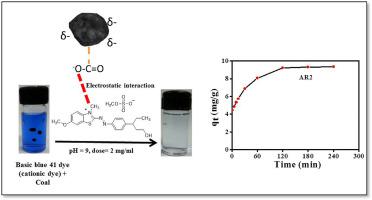
Coal is primarily a fuel material but lately it has been utilized as an adsorbent for removing toxic metal ions. However, its usage for removing organic pollutants is not well studied. We report here a systematic study on the use of coal samples of varying carbon contents as adsorbents for removing Basic Blue 41 as a model cationic dye. The coal samples were collected from coal mines and were thoroughly characterized. The concentrations of carbon, hydrogen, oxygen, nitrogen and sulphur contents were measured by CHNS analyzer. The concentrations of aluminum, silicon, sulphur, titanium and iron were determined by EDXRF, which corresponded to silicon dioxide (quartz) and aluminium silicate (kaolinite) as the major mineral inclusions, corroborated by XRD results and micrographs showing elemental maps determined from SEM-EDAX. The coal samples with low carbon content revealed higher adsorption capacity (qe ∼ 8.0–9.3 mg/g) of Basic Blue dye at optimized adsorbent dose (2 mg/mL), pH 9 and contact time (120 min). The adsorption kinetic studies satisfied pseudo second order model and the intra-particle diffusion of the dye was evident. The dye adsorption followed Langmuir adsorption isotherm, and the qmax values ranged between 17 and 30 mg/g for low carbon content coal. The FT-IR, Brunauer-Emmett-Teller (BET) surface area and zeta potential results of the coal samples could explain the adsorption phenomenon of cationic dye. The kinetic and thermodynamic studies revealed that the adsorption of Basic Blue 41 dye was based on chemisorptions mechanism.
中文翻译:

低碳煤的化学分析及其作为染料吸附剂的应用
煤主要是一种燃料材料,但最近它已被用作去除有毒金属离子的吸附剂。然而,它在去除有机污染物方面的用途还没有得到很好的研究。我们在此报告了一项关于使用不同碳含量的煤样品作为吸附剂去除作为模型阳离子染料的碱性蓝 41 的系统研究。煤样是从煤矿收集的,并进行了彻底的表征。碳、氢、氧、氮和硫含量的浓度由CHNS分析仪测量。铝、硅、硫、钛和铁的浓度由 EDXRF 测定,其对应于二氧化硅(石英)和硅酸铝(高岭石)作为主要矿物包裹体,由 XRD 结果和显微照片证实,显示元素图由 SEM-爱达克斯。q e ∼ 8.0–9.3 mg/g) 碱性蓝染料在优化的吸附剂剂量 (2 mg/mL)、pH 9 和接触时间 (120 分钟) 下。吸附动力学研究满足伪二级模型,染料的颗粒内扩散是明显的。染料吸附遵循朗缪尔吸附等温线,低碳含量煤的q最大值在 17 和 30 毫克/克之间。煤样的 FT-IR、Brunauer-Emmett-Teller (BET) 表面积和 zeta 电位结果可以解释阳离子染料的吸附现象。动力学和热力学研究表明,碱性蓝 41 染料的吸附基于化学吸附机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号