Chemical Geology ( IF 3.6 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.chemgeo.2021.120537 Hao Yu 1 , Peng Zhang 1 , Jiayu Liu 1 , Yunsong Zheng 1 , Nasiru Abba Mustapha 2, 3
|
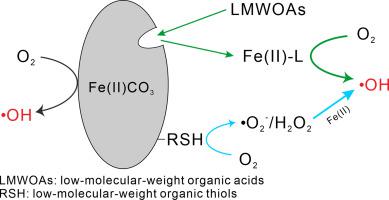
Oxygenation of siderite produces hydroxyl radicals (•OH) that are capable of transforming the pollutants. Yet, the influence of low-molecular-weight organic acids (LMWOAs)/ thiols (LMWOTs) which are widely occurring in soils and waters on •OH production from the oxygenation process of siderite is poorly understood. Results of this study show that the presence of LMWOAs/LMWOTs significantly facilitated •OH production from siderite oxidation by O2 at pH 6–8. For instance, at pH 7, the cumulative •OH concentration within 24 h was only 1.3 μM for oxygenation of 1 g/L siderite, while it boosted to 19.7 and 7.2 μM, respectively, with the respective addition of 1 mM ethylenediaminotetraacetate and cysteine. The mechanisms responsible for the enhanced •OH production are associated with the complexation ability and redox activity of LMWOAs/LMWOTs. In the absence of LMWOAs/LMWOTs, •OH is mainly produced from the oxidation of adsorbed Fe(II) by O2, followed by the oxidation of structural Fe(II) on siderite surface. In the presence of LMWOAs, LMWOAs react with adsorbed Fe(II) and structural Fe(II) to produce complexed Fe(II). Therefore, the oxidation of LMWOAs complexed Fe(II) by O2 predominantly contributed to •OH production. Although the complexation ability of LMWOTs is weaker than that of LMOWAs, LMWOTs can reduce O2 to generate •O2− and H2O2, thus facilitating •OH production. Based on the proposed mechanisms, a kinetic model was developed to quantify the relative contribution of each pathway to •OH production. The addition of 1 mM citrate and cysteine in siderite suspension could increase the removal of trichloroethylene from <5% to 15% and 7%, respectively. Our study reveals the overlooked catalytic effects of LMWOAs/LMWOTs in enhancing •OH production from siderite oxidation by O2 under redox-dynamic conditions.
中文翻译:

低分子量有机酸/硫醇对天然菱铁矿氧化产生羟基自由基的影响
菱铁矿的氧化产生能够转化污染物的羟基自由基 (•OH)。然而,人们对广泛存在于土壤和水中的低分子量有机酸 (LMWOA)/硫醇 (LMWOT) 对菱铁矿氧化过程产生的 •OH 的影响知之甚少。这项研究的结果表明,LMWOA/LMWOT 的存在显着促进了 O 2氧化菱铁矿产生 •OH在 pH 值 6–8 下。例如,在 pH 7 时,1 g/L 菱铁矿的氧化作用在 24 小时内累积的•OH 浓度仅为 1.3 μM,而在分别添加 1 mM 乙二氨基四乙酸盐和半胱氨酸后,它分别升至 19.7 和 7.2 μM。负责增强•OH 产生的机制与LMWOAs/LMWOTs 的络合能力和氧化还原活性有关。在没有 LMWOAs/LMWOTs 的情况下,•OH 主要是由 O 2氧化吸附的 Fe(II) 产生的,然后是菱铁矿表面结构 Fe(II) 的氧化。在 LMWOA 存在下,LMWOA 与吸附的 Fe(II) 和结构 Fe(II) 反应生成复合 Fe(II)。因此,LMWOAs 被 O 2氧化络合的 Fe(II)• OH 生产的主要贡献。虽然LMWOTs 的络合能力比LMOWAs 弱,但LMWOTs 可以还原O 2生成•O 2 -和H 2 O 2,从而促进•OH 的产生。基于所提出的机制,开发了一个动力学模型来量化每个途径对 •OH 生产的相对贡献。在菱铁矿悬浮液中加入 1 mM 柠檬酸盐和半胱氨酸可以将三氯乙烯的去除率分别从 <5% 增加到 15% 和 7%。我们的研究揭示了 LMWOAs/LMWOTs 在氧化还原动力学条件下通过 O 2氧化菱铁矿提高 •OH 产生的被忽视的催化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号