当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(V)/Iron(IV) species in graphitic carbon nitride-ferrate(VI)-visible light system: Enhanced oxidation of micropollutants
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.cej.2021.132610 Bao Pan 1, 2 , Mingbao Feng 1 , Jiani Qin 3 , Afzal Ahmed Dar 3 , Chuanyi Wang 3 , Xingmao Ma 4 , Virender K. Sharma 1
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.cej.2021.132610 Bao Pan 1, 2 , Mingbao Feng 1 , Jiani Qin 3 , Afzal Ahmed Dar 3 , Chuanyi Wang 3 , Xingmao Ma 4 , Virender K. Sharma 1
Affiliation
|
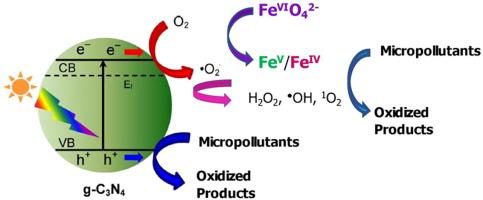
This paper investigated for the first time the visible light-induced oxidation of micropollutants using graphitic carbon nitride (g-CN) photocatalyst in the presence of ferrate(VI) (FeO) under mild alkaline conditions. The studied micropollutants have different molecular structures (i.e., carbamazepine (CBZ), sulfamethoxazole (SMX), sulfadimethoxine (SDM), trimethoprim (TMP), diclofenac (DCF), flumequine (FLU), atenolol (ATL), and caffeine (CAF)). Enhanced oxidation of all investigated micropollutants by the combined g-CN-FeO system was observed compared to g-CN or FeO alone under visible light irradiation. For example, 5.0 µM CBZ could be almost completely degraded in 5.0 min by the g-CN-FeO system. Comparatively, only ∼ 40% degradation of CBZ occurred by g-CN or FeO alone in 5.0 min under the same condition. Similar observations were found with other micropollutants, and the magnitude of enhancement varied with the structure of the micropollutants. The degradation experiments with and without oxygen in solution suggested the generation of O and O in g-CN-visible light system. However, with the presence of FeO in solution, the g-CN-visible light system generated high-valent iron-oxo intermediates (Fe/Fe) as the oxidizing species, evidenced by the selective conversion of methyl phenol oxide (PMSO) to PMSO, to cause increased oxidation of micropollutants. A plausible mechanism involving reactive species to oxidize micropollutants by the g-CN-FeO system under visible light irradiation was discussed. Oxidized products of CBZ were identified to reveal possible reaction pathways. The results of the g-CN-FeO-visible light system provided a new advanced oxidation process to synergistically degrade micropollutants in water efficiently.
中文翻译:

石墨氮化碳-高铁酸盐(VI)-可见光系统中的铁(V)/铁(IV)物质:增强微污染物的氧化
本文首次研究了在弱碱性条件下,在高铁酸盐(VI)(FeO)存在下,使用石墨氮化碳(g-CN)光催化剂对微污染物的可见光诱导氧化。研究的微污染物具有不同的分子结构(即卡马西平(CBZ)、磺胺甲恶唑(SMX)、磺胺二甲氧嘧啶(SDM)、甲氧苄啶(TMP)、双氯芬酸(DCF)、氟甲喹(FLU)、阿替洛尔(ATL)和咖啡因(CAF) )。在可见光照射下,与单独的 g-CN 或 FeO 相比,观察到组合的 g-CN-FeO 系统对所有研究的微污染物的氧化增强。例如,g-CN-FeO 系统可以在 5.0 分钟内几乎完全降解 5.0 µM CBZ。相比之下,在相同条件下,单独使用 g-CN 或 Fe3O 在 5 分钟内仅降解 CBZ 约 40%。在其他微污染物中也发现了类似的结果,并且增强的幅度随微污染物的结构而变化。溶液中含氧和不含氧的降解实验表明,g-CN-可见光体系中产生了O和O。然而,当溶液中存在 Fe3O 时,g-CN-可见光系统会产生高价铁氧中间体 (Fe/Fe) 作为氧化物质,这一点可以通过甲基苯酚氧化物 (PMSO) 选择性转化为 PMSO 来证明。 ,导致微污染物氧化增加。讨论了可见光照射下 g-CN-FeO 体系中活性物质氧化微污染物的合理机制。鉴定了 CBZ 的氧化产物以揭示可能的反应途径。 g-CN-FeO-可见光系统的结果提供了一种新的高级氧化工艺,可以有效地协同降解水中的微污染物。
更新日期:2021-09-23
中文翻译:

石墨氮化碳-高铁酸盐(VI)-可见光系统中的铁(V)/铁(IV)物质:增强微污染物的氧化
本文首次研究了在弱碱性条件下,在高铁酸盐(VI)(FeO)存在下,使用石墨氮化碳(g-CN)光催化剂对微污染物的可见光诱导氧化。研究的微污染物具有不同的分子结构(即卡马西平(CBZ)、磺胺甲恶唑(SMX)、磺胺二甲氧嘧啶(SDM)、甲氧苄啶(TMP)、双氯芬酸(DCF)、氟甲喹(FLU)、阿替洛尔(ATL)和咖啡因(CAF) )。在可见光照射下,与单独的 g-CN 或 FeO 相比,观察到组合的 g-CN-FeO 系统对所有研究的微污染物的氧化增强。例如,g-CN-FeO 系统可以在 5.0 分钟内几乎完全降解 5.0 µM CBZ。相比之下,在相同条件下,单独使用 g-CN 或 Fe3O 在 5 分钟内仅降解 CBZ 约 40%。在其他微污染物中也发现了类似的结果,并且增强的幅度随微污染物的结构而变化。溶液中含氧和不含氧的降解实验表明,g-CN-可见光体系中产生了O和O。然而,当溶液中存在 Fe3O 时,g-CN-可见光系统会产生高价铁氧中间体 (Fe/Fe) 作为氧化物质,这一点可以通过甲基苯酚氧化物 (PMSO) 选择性转化为 PMSO 来证明。 ,导致微污染物氧化增加。讨论了可见光照射下 g-CN-FeO 体系中活性物质氧化微污染物的合理机制。鉴定了 CBZ 的氧化产物以揭示可能的反应途径。 g-CN-FeO-可见光系统的结果提供了一种新的高级氧化工艺,可以有效地协同降解水中的微污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号